- Language:English
- English
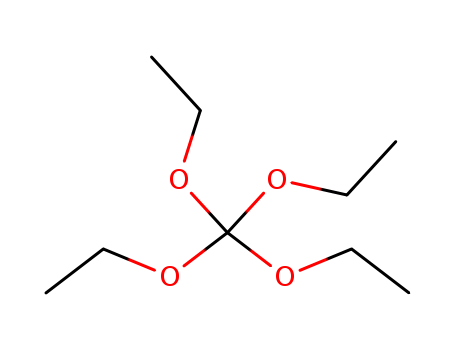

CasNo: 78-09-1
Molecular Formula: C9H20O4
Appearance: clear colorless liquid
|
Chemical Properties |
clear colorless liquid |
|
Uses |
Tetraethyl Orthocarbonate is used in the synthesis of chemokine receptor-5 inhibitors against HIV-1, benzobisoxazoles and in the preparation of organic semiconductors. It is also employed in the synthesis of 2,7-dimethylene-1,4,6,9-tetraoxaspiro[4,4]nonane and in the synthesis of cross linked poly(orthocarbonate)s used as organic solvent absorbent. |
|
Purification Methods |
Likely impurities are hydrolysis products. Shake the orthocarbonate with brine (saturated NaCl, dilute with a little Et2O if amount of material is small) and dry (MgSO4). The organic layer is filtered off and evaporated, and the residue is distilled through a helices packed fractionating column with a total reflux partial take-off head. All distillations can be done at atmospheric pressure in an inert atmosphere (e.g. N2). [Roberts & McMahon Org Synth Coll Vol IV 457 1963, Connolly & Dyson J Chem Soc 828 1937, Tieckelmann & Post J Org Chem 13 266 1948, for review see Kantlehner et al. Justus Liebigs Ann Chem 507 207 1982, Beilstein 3 IV 6.] |
InChI:InChI=1/C9H20O4/c1-5-10-9(11-6-2,12-7-3)13-8-4/h5-8H2,1-4H3
The invention relates to the field of re...
The invention relates to a synthesis met...
An unexpected modulation of the chemosel...
A process for producing crystals of 2-et...
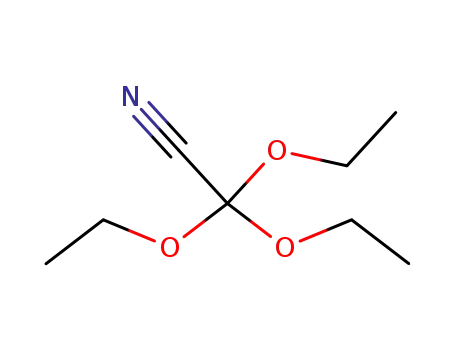
triethoxyacetonitrile

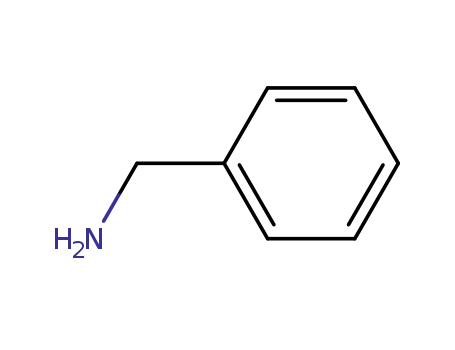
benzylamine

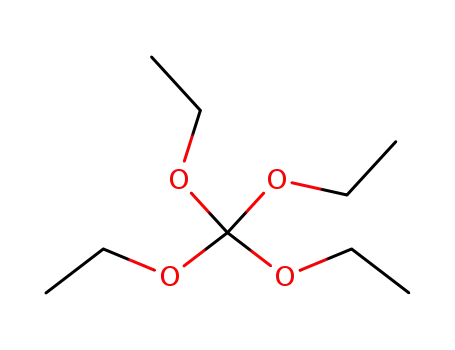
orthocarbonic acid tetraethyl ester

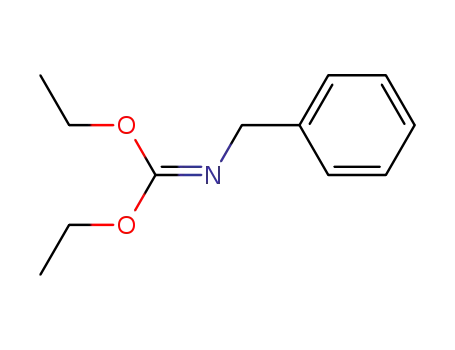
Diethyl(benzylimido)carbonat

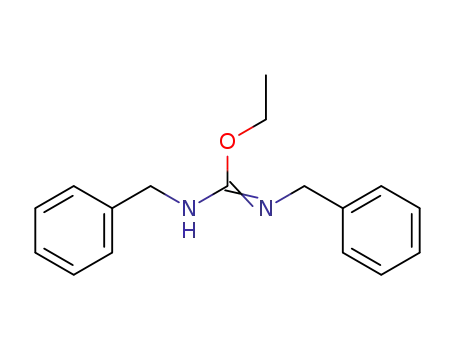
1,3-Dibenzyl-2-ethylisoharnstoff
| Conditions | Yield |
|---|---|
|
at 120 ℃;
|
14% 45% |
|
at 120 ℃;
Heating;
|
45% 14% |
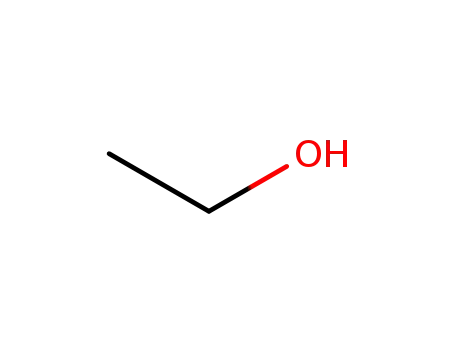
ethanol

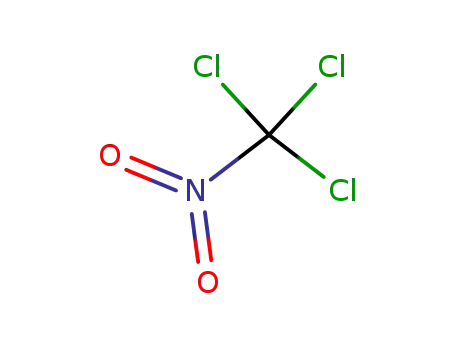
chloropicrin


orthocarbonic acid tetraethyl ester
| Conditions | Yield |
|---|---|
|
With
sodium ethanolate;
at 65 - 70 ℃;
for 3h;
Temperature;
Large scale;
|
91% |
|
With
sodium;
Darst.;
|
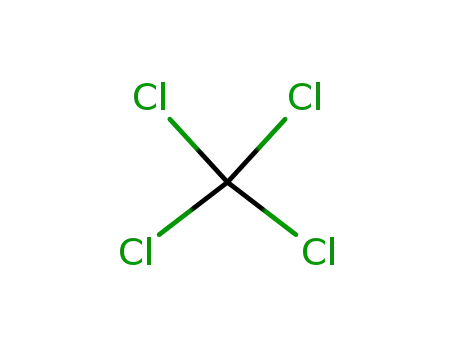
tetrachloromethane
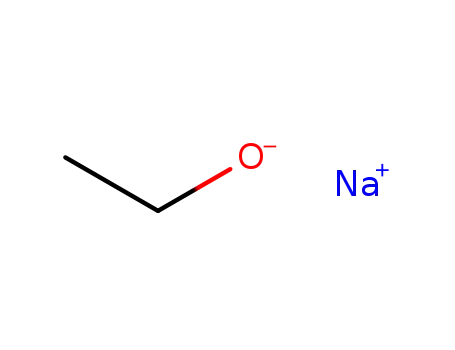
sodium ethanolate
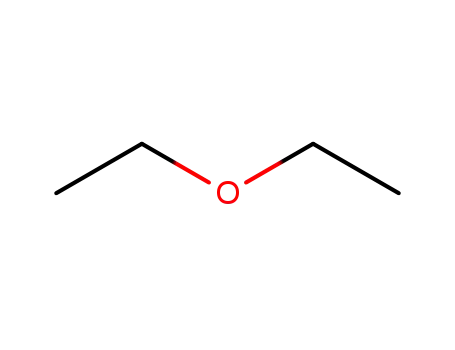
diethyl ether
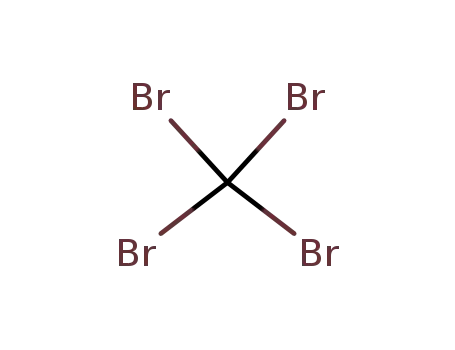
carbon tetrabromide
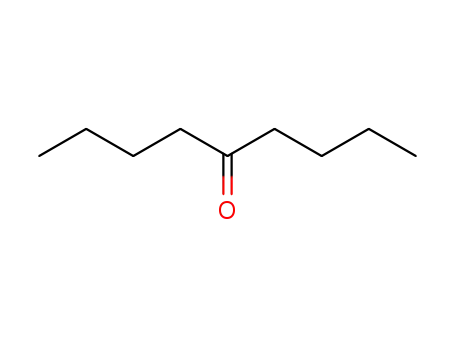
nonanone-5
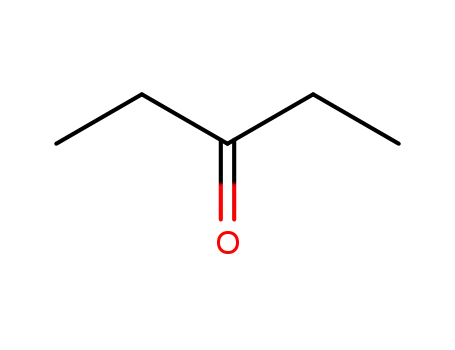
pentan-3-one
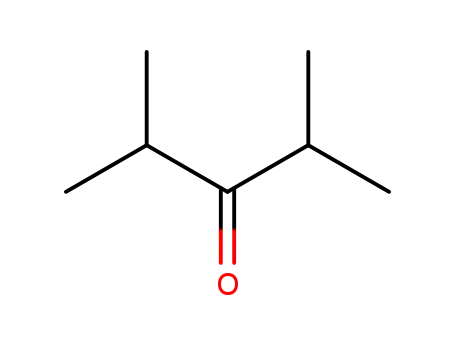
2,4-dimethylpentan-3-one
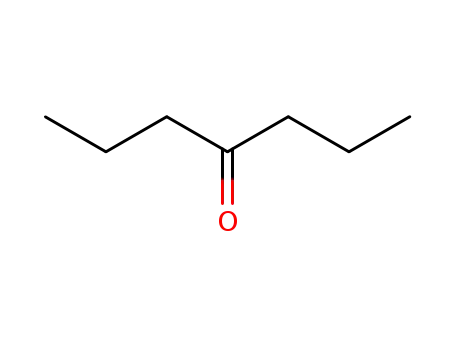
4-heptanone
CAS:138071-82-6
CAS:57280-22-5
CAS:1663-61-2
CAS:919-29-9