- Language:English
- English
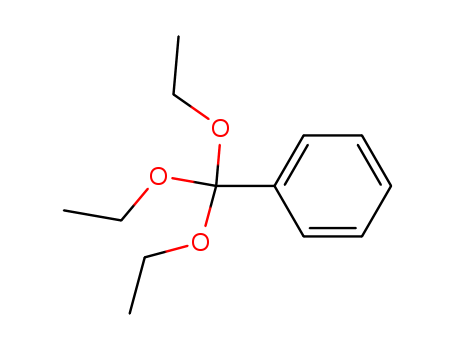

CasNo: 1663-61-2
Molecular Formula: C13H20O3
Appearance: White crystalline power
|
Chemical Properties |
clear colorless liquid |
|
Uses |
Triethyl Orthobenzoate can be used as a thermal additive to air conditioning refrigerant oil. This compound can also be used as a catalyst for olefin polymerization. |
InChI:InChI=1/C13H20O3/c1-4-14-13(15-5-2,16-6-3)12-10-8-7-9-11-12/h7-11H,4-6H2,1-3H3
The method is characterized in that the ...
A new electrochemical methodology has be...
Dynamic adaptability and biodegradabilit...
Reversible covalent reactions have becom...
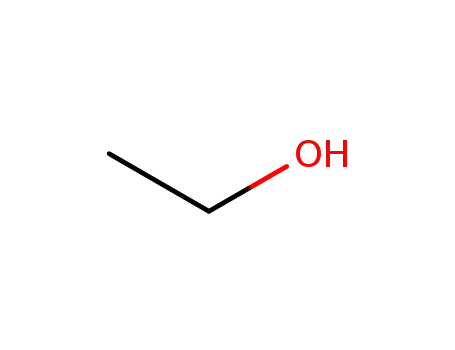
ethanol

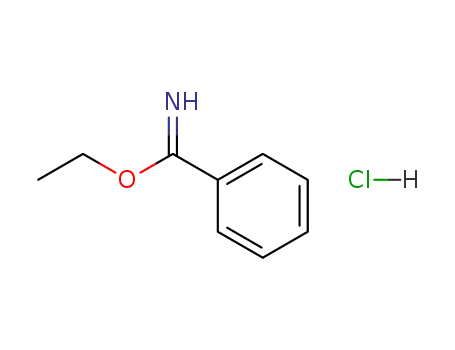
ethyl benzimidate hydrochloride


benzamide

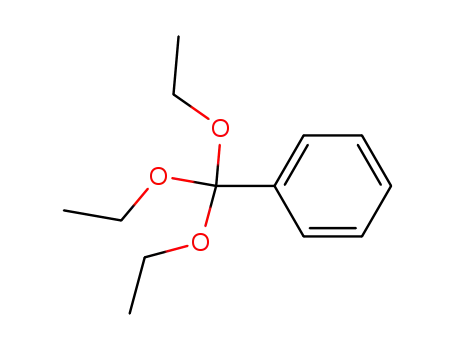
triethoxymethylbenzene
| Conditions | Yield |
|---|---|
|
|
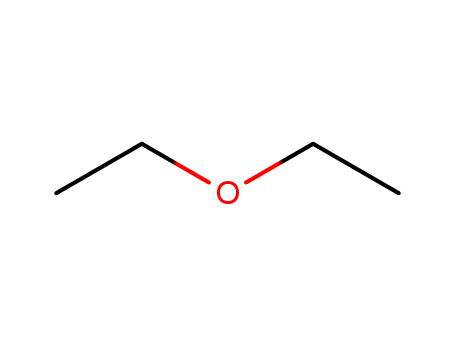
diethyl ether

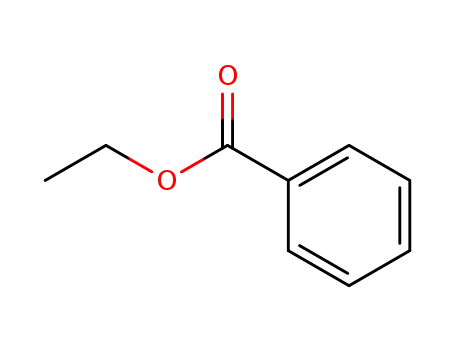
benzoic acid ethyl ester


triethoxymethylbenzene
| Conditions | Yield |
|---|---|
|
With
boron trifluoride;
In
Hexadecane;
at 110 ℃;
for 6h;
Temperature;
Reagent/catalyst;
Time;
Autoclave;
|
95% |

ethanol

ethyl benzimidate hydrochloride
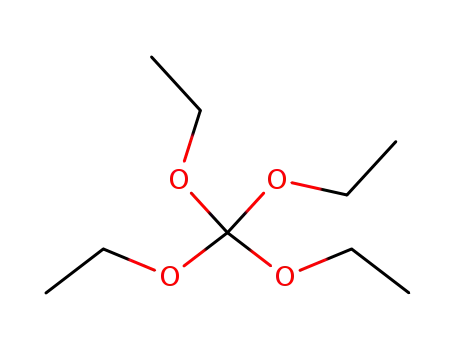
orthocarbonic acid tetraethyl ester

diethyl ether
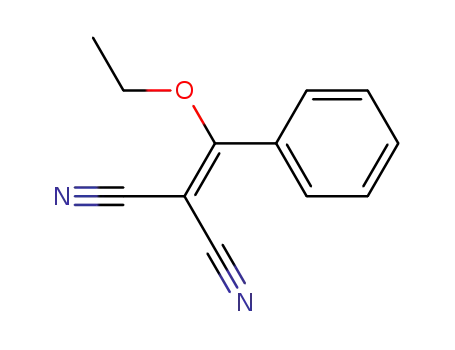
2-(ethoxy(phenyl)methylene)malononitrile
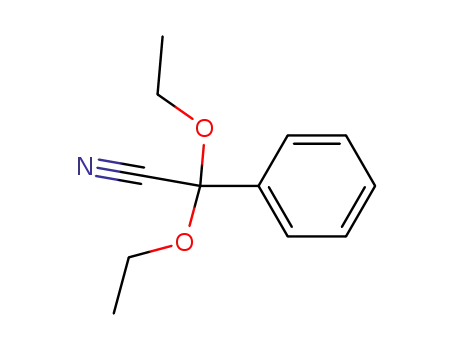
α,α-diethoxybenzeneacetonitrile
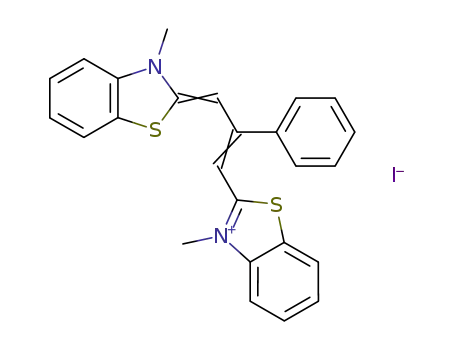
3,3′-dimethyl-9-phenylthiacarbocyanine iodide
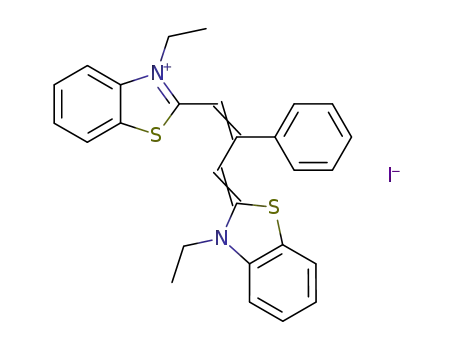
1,3-bis-(3-ethyl-benzothiazol-2-yl)-2-phenyl trimethinium ; iodide
CAS:138071-82-6
CAS:57280-22-5
CAS:52698-46-1
CAS:78-09-1