- Language:English
- English
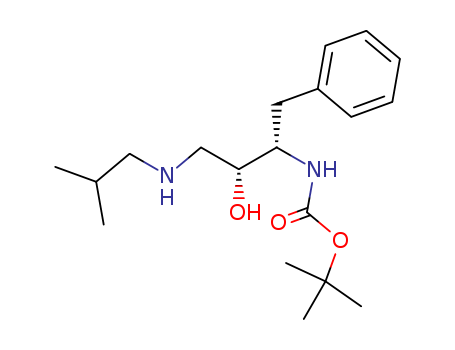

CasNo: 160232-08-6
Molecular Formula: C19H32N2O3
Appearance: White solid
InChI:InChI=1/C19H32N2O3/c1-14(2)12-20-13-17(22)16(11-15-9-7-6-8-10-15)21-18(23)24-19(3,4)5/h6-10,14,16-17,20,22H,11-13H2,1-5H3,(H,21,23)/t16-,17+/m0/s1
(S)-3-tert-Butoxycarbonylamino-1-nitro-2...
New heteroaryl HIV-protease inhibitors b...
A robust and safe industrial process, in...
A novel class of HIV-1 protease inhibito...
The invention provides a nitrogen-contai...
Upon the basis of both possible ligand-b...
Flexible heterocyclic moieties as the P2...
isobutylamine

tert-butyl ((2S,3S)-4-chloro-3-hydroxy-1-phenylbutan-2-yl)carbamate

(2R,3S)-3-tert-butoxycarbonylamino-1-isobutylamino-4-phenyl-2-butanol
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In ethanol; at 15 - 20 ℃; Large scale;
|
97.3% |
|
With sodium carbonate; In water; at 60 - 65 ℃; for 3h;
|
105 g |
|
With sodium carbonate; In water; at 60 - 65 ℃; for 3h;
|
105 g |
isobutylamine

(1-oxiranyl-2-phenylethyl)carbamic acid tert-butyl ester

(2R,3S)-3-tert-butoxycarbonylamino-1-isobutylamino-4-phenyl-2-butanol
| Conditions | Yield |
|---|---|
|
In isopropyl alcohol; at 20 ℃;
|
100% |
|
In isopropyl alcohol; at 60 ℃; for 6h;
|
100% |
|
In isopropyl alcohol; at 60 ℃; Inert atmosphere;
|
100% |
|
In isopropyl alcohol; for 6h; Heating;
|
99% |
|
In ethanol; at 78 ℃; for 1h; Inert atmosphere;
|
99.8% |
|
In isopropyl alcohol; at 90 ℃; for 72h;
|
99% |
|
In isopropyl alcohol; at 80 ℃; for 3h;
|
97% |
|
at 65 - 75 ℃; for 3h;
|
97.63% |
|
at 65 - 70 ℃;
|
97.87% |
|
In neat (no solvent); at 50 - 60 ℃; for 2h; Solvent; Reflux;
|
95.7% |
|
In ethanol; at 20 - 80 ℃; for 3h; Inert atmosphere;
|
91% |
|
In acetonitrile; for 6h; Reflux;
|
91.5% |
|
In acetonitrile; for 6h; Reflux;
|
91.5% |
|
In isopropyl alcohol; at 50 ℃; for 5h;
|
90% |
|
In ethanol; at 80 ℃; for 3h;
|
85% |
|
In acetonitrile; at 80 ℃; for 5h;
|
83% |
|
In acetonitrile; at 80 ℃; for 5h;
|
83% |
|
In acetonitrile; at 80 ℃; for 5h;
|
83% |
|
In acetonitrile; at 80 ℃; for 6h; Inert atmosphere; Sealed tube;
|
83% |
|
In acetonitrile; at 80 ℃; for 6h;
|
83% |
|
In isopropyl alcohol; at 68 ℃; for 3.5h;
|
|
|
In isopropyl alcohol; for 16h; Heating;
|
|
|
In dichloromethane;
|
|
|
In isopropyl alcohol; at 60 ℃;
|
|
|
In isopropyl alcohol;
|
|
|
In isopropyl alcohol; at 80 ℃; for 2h;
|
|
|
In ethanol; at 80 ℃; for 3h;
|
|
|
In ethanol; at 50 ℃;
|
|
|
Heating / reflux;
|
|
|
|
|
|
at 80 ℃;
|
|
|
In ethanol; at 80 ℃; for 3h;
|
|
|
In isopropyl alcohol; for 1h; Heating / reflux;
|
|
|
In ethanol; at 80 ℃; for 3h; Inert atmosphere;
|
|
|
In methanol; for 3h; Reflux;
|
|
|
In dichloromethane; for 3h; Reflux;
|
|
|
at 70 - 75 ℃; for 3h;
|
231 g |
|
at 70 - 75 ℃; for 3h;
|
231 g |
|
In ethanol; at 80 ℃; for 3h;
|
|
|
In isopropyl alcohol;
|
|
|
In isopropyl alcohol; at 80 ℃; for 3h;
|
|
|
In acetonitrile; at 80 ℃; for 6h;
|
|
|
In acetonitrile; at 80 ℃; for 6h; Inert atmosphere;
|
C19H31N3O4
isobutyraldehyde
(1S,2R)-(3-amino-1-benzyl-2-hydroxy-propyl)-carbamic acid tert butyl ester
isobutylamine
tert-butyl (1S,2R)-1-benzyl-2-hydroxy-3-{isobutyl[(4-methoxyphenyl)sulfonyl]amino}propylcarbamate
[(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester
(S)-2-[3-((2R,3S)-3-tert-Butoxycarbonylamino-2-hydroxy-4-phenyl-butyl)-3-isobutyl-ureido]-4-methyl-pentanoic acid methyl ester
tert-butyl ((2S,3R)-4-((4-acetyl-N-isobutylphenyl)sulfonamido)-3-hydroxy-1-phenylbutan-2-yl)carbamate
CAS:138071-82-6
CAS:38083-17-9
CAS:870-46-2