- Language:English
- English
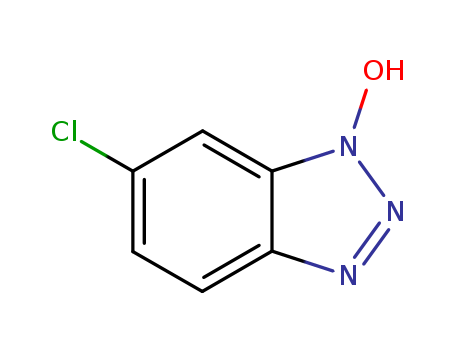

CasNo: 26198-19-6
Molecular Formula: C6H4 Cl N3 O
|
Chemical Properties |
White powder |
InChI:InChI=1/C6H4ClN3O/c7-4-1-2-5-6(3-4)10(11)9-8-5/h1-3,11H
A molybdenum-catalyzed deoxygenation of ...
The N-heterocyclic carbene and hydroxami...
1-hydroxybenzotriazole derivatives are u...
The reaction of N,N-diethyl carbamates o...
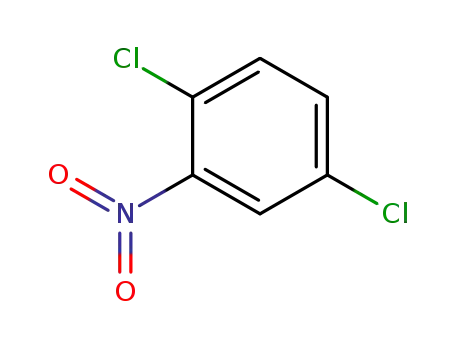
2,5-dichloronitrobenzene

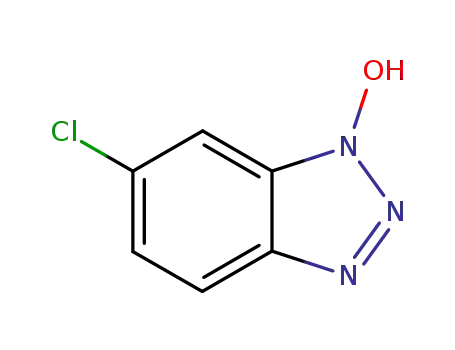
6-chloro-1-hydroxybenzotriazole
| Conditions | Yield |
|---|---|
|
With
hydrazine hydrate;
In
ethanol;
for 36h;
Reflux;
|
79% |
|
With
hydrazine hydrate;
In
ethanol;
for 24h;
Reflux;
|
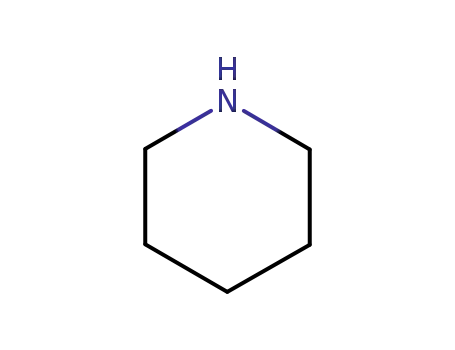
piperidine

![6-chloro-1H-benzo[d][1,2,3]triazol-1-yl diethylcarbamate](/upload/2023/6/24d69cbe-b8b9-4291-8d52-26adce943d65.png)
6-chloro-1H-benzo[d][1,2,3]triazol-1-yl diethylcarbamate

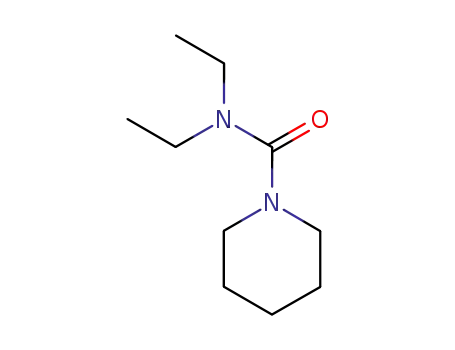
N,N-diethylpiperidine-4-carboxamide


6-chloro-1-hydroxybenzotriazole
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
at 30 ℃;
Temperature;
Kinetics;
|

piperidine
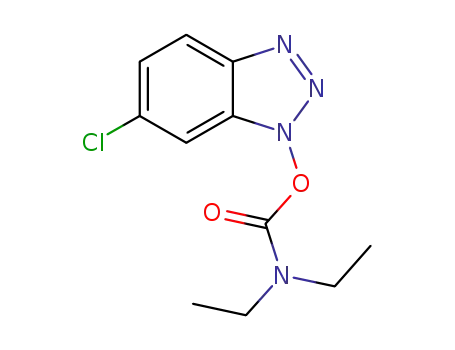
6-chloro-1H-benzo[d][1,2,3]triazol-1-yl diethylcarbamate
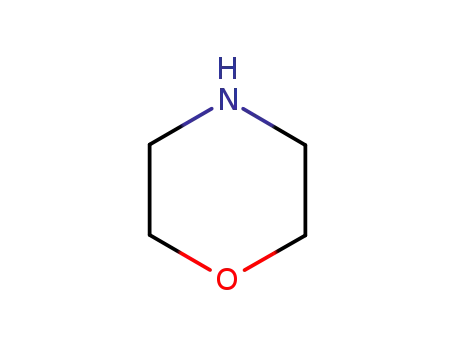
morpholine

2,5-dichloronitrobenzene
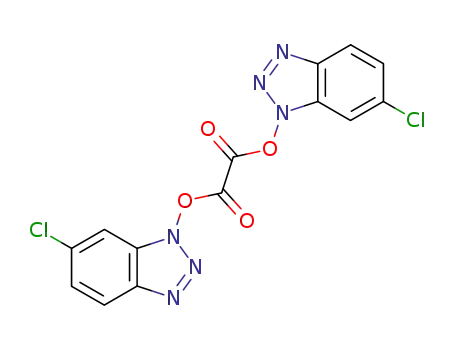
1,1'-di(6-chlorobenzotriazolo) oxallate(Cl-DBTO)
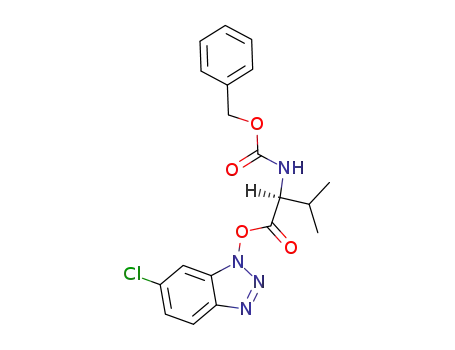
(S)-2-Benzyloxycarbonylamino-3-methyl-butyric acid 6-chloro-benzotriazol-1-yl ester
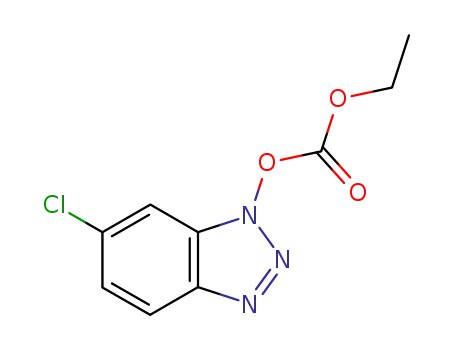
6-chloro-1H-benzo[d][1,2,3]triazol-1-yl ethyl carbonate
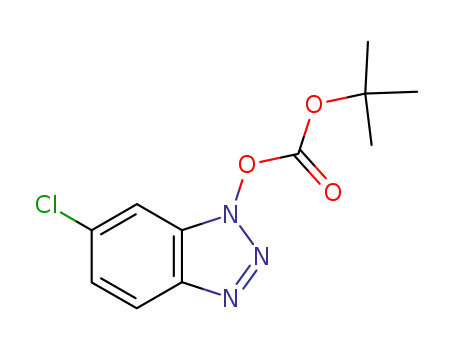
1-tert-butoxycarbonyloxy-6-chloro-1H-benzotriazole
CAS:38083-17-9
CAS:120011-70-3
CAS:577-16-2
CAS:538-75-0