- Language:English
- English
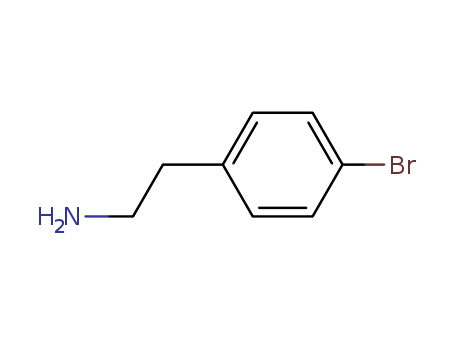

CasNo: 73918-56-6
Molecular Formula: C8H10BrN
Appearance: Clear colourless to yellow liquid
|
Chemical Properties |
CLEAR COLOURLESS TO YELLOW LIQUID |
|
Uses |
p-Bromophenethylamine was used in the synthesis of pyrazinoisoquinoline derivatives and N-2-(4-bromophenyl)ethyl chloroacetamide. It was also used in the synthesis of alkyl arylamino sufides employing elemental sulfur and various halides. |
IUPAC Name: 2-(4-bromophenyl)ethanamine
Isomeric SMILES: C1=CC(=CC=C1CCN)Br
InChIKey: ZSZCXAOQVBEPME-UHFFFAOYSA-N
InChI: InChI=1S/C8H10BrN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5-6,10H2
In our work, we prepare 2D Ruddlesden–Popper perovskites based on 4-bromophenylethylammonium (BPEA) using a new additive of ionic liquids MAAc (methylammonium acetate). It is found that an intermediate perovskite with distorted [PbI6−xAcx]4− octahedron is formed with the addition of MAAc, making the quality and photoelectric properties of the resultant films greatly enhanced.
Nitrogen-containing motifs are widely pr...
Experiments with whole cells and cell extracts revealed that 4-bromo- and 4-iodobenzoate were metabolized like 4-chlorobenzoate, involving an initial hydrolytic dehalogenation yielding 4-hydroxybenzoate, which in turn was hydroxylated to 3,4-dihydroxybenzoate.
p-bromophenethylamine, an odorless liquid. The unique isotopic signature of the Br introduced facilitated definitive localization of phosphorylation sites in multiphosphorylated peptides with highly adjacent serine or threonine residues.
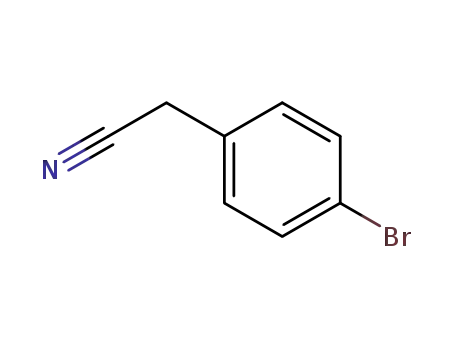
4-Bromophenylacetonitrile

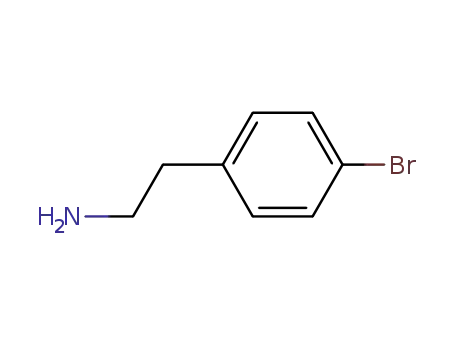
4-Bromophenethylamine
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; {[κ3-(1-pz)2HB(N=CHCH3)]Ru(cymene)}+ TfO-; sodium t-butanolate; In methanol; at 70 ℃; for 14h;
|
85% |
|
With methanol; sodium tetrahydroborate; C18H25BN5Ru(1+)*CF3O3S(1-); sodium t-butanolate; for 14h; Reagent/catalyst; Reflux;
|
85% |
|
With samarium diiodide; water; triethylamine; In tetrahydrofuran; at 20 ℃; for 0.0833333h; Inert atmosphere;
|
83% |
|
4-Bromophenylacetonitrile; With borane-THF; In tetrahydrofuran; at 0 ℃; for 24.5h; Reflux;
for 4h; Reflux;
|
80% |
|
4-Bromophenylacetonitrile; With borane-THF; 5-bromoisoindoline; In tetrahydrofuran; at 75 ℃;
With hydrogenchloride; water; In tetrahydrofuran; at 20 ℃;
|
78% |
|
With lithium aluminium tetrahydride; diethyl ether;
|
|
|
With lithium aluminium tetrahydride;
|
|
|
With dimethylsulfide borane complex; In tetrahydrofuran; Heating;
|
|
|
With borane-THF; In tetrahydrofuran; for 8h; Reflux;
|

1-bromo-4-ethenyl-benzene


4-Bromophenethylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: NOCl, HCl / diethyl ether
2: Et3N / diethyl ether
3: LiAlH4 / diethyl ether
With hydrogenchloride; lithium aluminium tetrahydride; nitrosylchloride; triethylamine; In diethyl ether;
|
|
|
With D-Glucose; ammonia; oxygen; In aq. phosphate buffer; at 30 ℃; for 24h; regioselective reaction; Green chemistry;
|
|
|
Multi-step reaction with 3 steps
1: oxygen; styrene monooxygenase / Enzymatic reaction
2: styrene oxide isomerase / Enzymatic reaction
3: ammonia; ω-transaminase; L-alanine dehydrogenase / Enzymatic reaction
With styrene monooxygenase; L-alanine dehydrogenase; ω-transaminase; styrene oxide isomerase; ammonia; oxygen;
|
|
|
With 3,6‐di‐tert‐butyl‐9‐mesityl‐10‐phenylacridin‐10‐ium tetrafluoroborate; ammonium carbonate; 2-amino-benzenethiol; In dichloromethane; acetonitrile; at 20 ℃; for 12h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation;
|
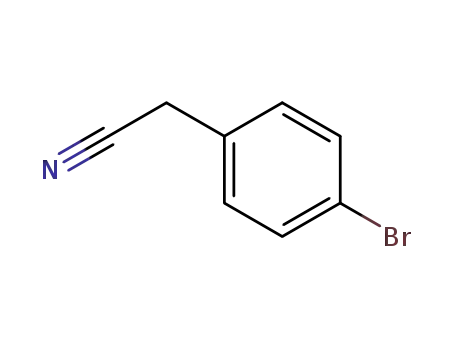
4-Bromophenylacetonitrile
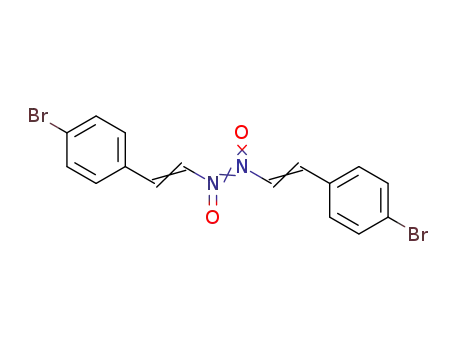
Bis-(β-nitroso-4-brom-styrol)
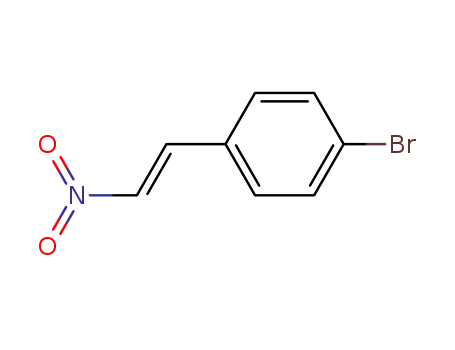
(E)-1-bromo-4-(2-nitrovinyl)benzene
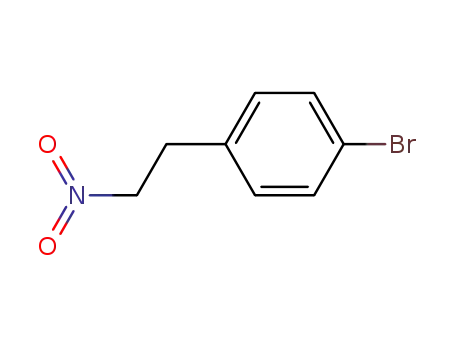
1-bromo-4-(2-nitroethyl)benzene
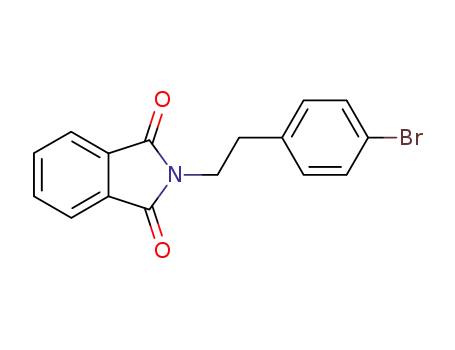
2-(2-(4-bromophenyl)ethyl)isoindolin-1,3-dione
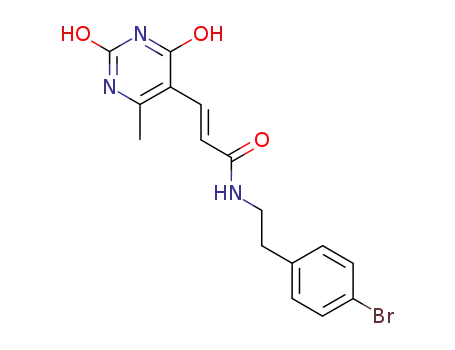
(E)-N-[2-(4-Bromo-phenyl)-ethyl]-3-(2,4-dihydroxy-6-methyl-pyrimidin-5-yl)-acrylamide
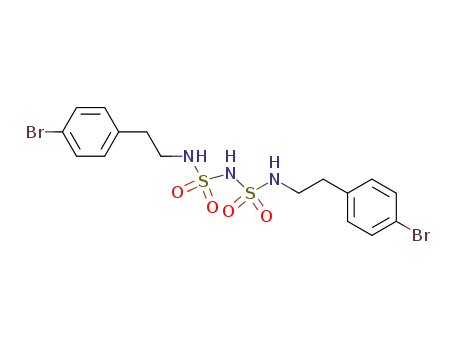
C16H19Br2N3O4S2
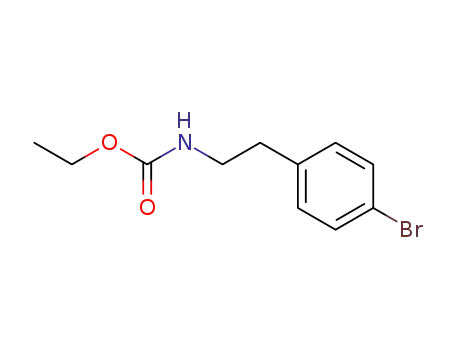
ethyl <2-(4-bromophenyl)ethyl>carbamate
CAS:1953-04-4
CAS:20826-04-4
CAS:58971-11-2
CAS:13078-79-0