- Language:English
- English
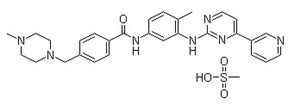

CasNo: 220127-57-1
Molecular Formula: C29H31N7O.CH4O3S
Appearance: Off-white solid
|
Description |
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. Imatinib mesylate (Gleevec) is a small-molecule inhibitor of the fusion protein Bcr-Abl, the causal agent in chronic myelogenous leukemia. |
|
Chemical Properties |
Off-White Solid |
|
Originator |
Novartis (Switzerland) |
|
Uses |
Imatinib mesylate, a protein tyrosine kinase valuable inhibitor in the treatment of chronic myelogenous leukemia and gastrointestinal, is a rationally designed oral signal transduction inhibitor that specifically targets several protein tyrosine kinases, Abl, Arg (Abl-related gene), the stem-cell factor receptor (c-KIT), platelet-derived growth factor receptor (PDGF-R), and their oncogenic forms, most notably Bcr-Abl. Imatinib has been shown to have remarkable clinical activity in patients with chronic myeloid leukemia (CML) and malignant gastrointestinal stromal tumors (GIST). |
|
Definition |
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours. |
|
Brand name |
Gleevec, Glivec |
|
Biochem/physiol Actions |
Imatinib mesylate is a tyrosine kinase inhibitor with antineoplastic activity. Imatinib is a potent inhibitor of the Bcr-Abl kinase encoded by the bcr-abl oncogene as well as receptor tyrosine kinases encoded by c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes. Imatinib mesylate inhibition of Bcr-Abl tyrosine kinase created by the Philadelphia chromosome abnormality found in CML decreases proliferation and enhances apoptosis in leukemias CML and ALL. Inhibition of c-kit tyrosine activity inhibits mast-cell and cellular proliferation in those diseases overexpressing c-kit such as gastrointestinal stromal tumor (GIST). |
IUPAC Name: methanesulfonic acid;4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide
Isomeric SMILES: CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.CS(=O)(=O)O
InChIKey: YLMAHDNUQAMNNX-UHFFFAOYSA-N
InChI: InChI=1S/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4)
We conducted an open-label, randomized, multicenter trial to evaluate the activity of imatinib in patients with advanced gastrointestinal stromal tumor. A total of 147 patients were randomly assigned to receive 400 mg or 600 mg of imatinib daily.
Imatinib induced major cytogenetic responses in 60 percent of the 454 patients with confirmed chronic-phase CML and complete hematologic responses in 95 percent. Imatinib induced high rates of cytogenetic and hematologic responses in patients with chronic-phase CML in whom previous interferon therapy had failed.
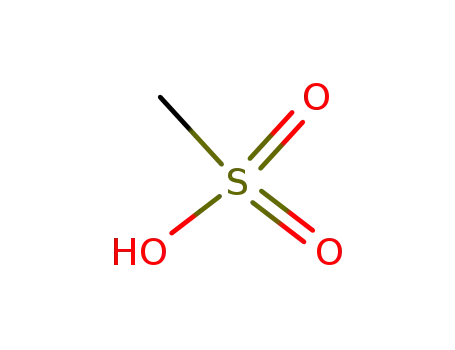
methanesulfonic acid

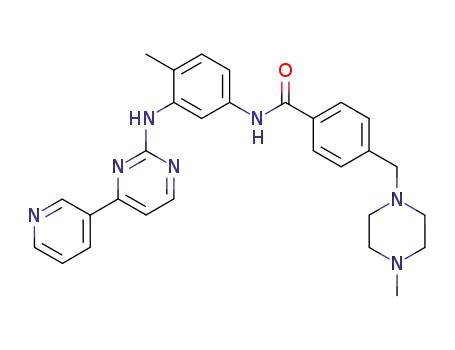
imatinib

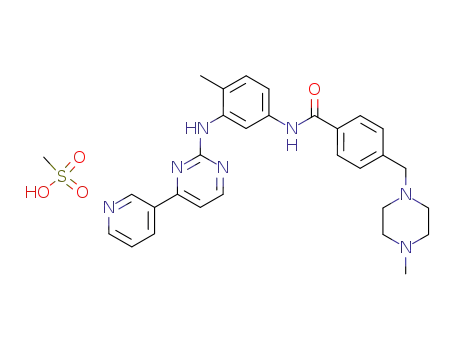
imatinib mesylate
| Conditions | Yield |
|---|---|
|
In acetone; at 55 - 60 ℃; for 2h; Concentration; Temperature; Time;
|
99.89% |
|
In di-isopropyl ether; isopropyl alcohol; at 20 ℃; for 3.16667h; Product distribution / selectivity; Reflux;
|
97% |
|
In isopropyl alcohol; acetone; for 3h; Reflux;
|
97.1% |
|
In isopropyl alcohol; at 65 - 75 ℃; Product distribution / selectivity;
|
96.3% |
|
In water; isopropyl alcohol; at 20 ℃; Product distribution / selectivity;
|
96.6% |
|
In isopropyl alcohol; at 70 ℃; for 0.5h;
|
96% |
|
In 1,2-dimethoxyethane; tert-butyl methyl ether; at 10 - 40 ℃; Product distribution / selectivity;
|
95% |
|
In 1,2-dimethoxyethane; tert-butyl methyl ether; at 10 - 40 ℃; Product distribution / selectivity;
|
95% |
|
In ethyl acetate; at 40 - 60 ℃; Product distribution / selectivity;
|
95% |
|
In chloroform; at 50 ℃; for 5h; Temperature;
|
95% |
|
In acetonitrile; at 25 - 30 ℃; for 0.5h;
|
94% |
|
In 4-methyl-2-pentanone; acetone; at 60 - 65 ℃; for 3.5h; Product distribution / selectivity;
|
94% |
|
In chloroform; water; isopropyl alcohol; at 60 - 70 ℃;
|
94.9% |
|
In ethanol; isopropyl alcohol; at 24 - 70 ℃; for 0.166667h; Product distribution / selectivity;
|
93.6% |
|
In nitromethane; at 90 ℃; Product distribution / selectivity;
|
93% |
|
In isopropyl alcohol; at 74 - 80 ℃; for 1h; Solvent; Temperature;
|
93% |
|
In 4-methyl-2-pentanone; at 65 ℃; for 4h; Product distribution / selectivity;
|
92% |
|
In ethanol; at 60 - 78 ℃; for 1.33h; Autoclave;
|
92.6% |
|
In acetone; at 20 ℃; for 1.5h; Product distribution / selectivity;
|
90% |
|
In acetonitrile; at 15 ℃; for 5h; Product distribution / selectivity;
|
90% |
|
In ethanol; at -40 - -28 ℃; for 14.5h; Product distribution / selectivity;
|
90.1% |
|
In ethanol; water; at -10 - -5 ℃; for 0.333333h;
|
89% |
|
In ethanol; water; at -10 - -5 ℃; for 0.333333h; Product distribution / selectivity;
|
89% |
|
In acetonitrile; at 20 ℃; for 3.5h; Product distribution / selectivity;
|
88% |
|
In dimethyl sulfoxide; at 40 - 45 ℃; Product distribution / selectivity;
|
88% |
|
In isopropyl alcohol; for 3h; Reflux;
|
88% |
|
In acetonitrile; at 20 ℃; for 2.75h; Product distribution / selectivity; Heating / reflux;
|
86.6% |
|
In butanone; at 75 - 77 ℃; for 2h; Product distribution / selectivity;
|
86.5% |
|
In methanol; isopropyl alcohol; at 20 - 85 ℃; for 7.5h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In acetone; at 20 ℃; for 1.75h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In acetone; at 20 ℃; for 1.75h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In ethanol; water; at -27 - -5 ℃; Product distribution / selectivity;
|
85% |
|
In water; isopropyl alcohol; at -28 - -1 ℃; for 0.333333h; Product distribution / selectivity;
|
85% |
|
In ethanol; tert-butyl methyl ether; water; at -27 - -5 ℃; for 3.41667 - 4.75h; Product distribution / selectivity;
|
85% |
|
In methanol; dichloromethane; at 20 ℃; for 3.83333h; Product distribution / selectivity; Heating / reflux;
|
83% |
|
In propan-1-ol; acetic acid; at 82 ℃; under 15.0015 Torr; Product distribution / selectivity;
|
83% |
|
In dimethyl sulfoxide; isopropyl alcohol; at 90 - 95 ℃;
|
80% |
|
In tetrahydrofuran; at 10 - 20 ℃; for 0.666667h; Product distribution / selectivity;
|
76% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 1.91667 - 2.25h; Product distribution / selectivity;
|
72% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 0.333333 - 1.58333h; Product distribution / selectivity;
|
71% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 1.58333h; Product distribution / selectivity;
|
71% |
|
In water; isopropyl alcohol; at 20 ℃; for 0.5h; Product distribution / selectivity;
|
68% |
|
In butan-1-ol; at 70 - 80 ℃; pH=4.5; Inert atmosphere;
|
65.7% |
|
In butan-1-ol; at 70 - 80 ℃; pH=4.5;
|
65.7% |
|
In dioxolane, 1,3-; water; at 5 - 20 ℃; for 0.1667 - 6.25h; Product distribution / selectivity;
|
50% |
|
In ethanol; at 65 - 76 ℃; for 0.0833333 - 0.25h; Product distribution / selectivity;
|
49.1% |
|
In chloroform; at 20 - 50 ℃; for 5 - 41h;
|
|
|
In dichloromethane; at 20 ℃; for 5h;
|
|
|
In tert-butyl alcohol; at 70 - 80 ℃; for 1h; Product distribution / selectivity;
|
|
|
In propan-1-ol; at 70 - 72 ℃; for 0.0833333h; Product distribution / selectivity;
|
|
|
In isopropyl alcohol; for 2h; Heating / reflux;
|
|
|
In 1-methylcyclohexan-4-one; at 65 ℃; for 4h; Product distribution / selectivity;
|
|
|
In ethanol; at 65 ℃; Product distribution / selectivity;
|
|
|
In 1-methylcyclohexan-4-one; at 65 ℃;
|
|
|
In methanol;
|
|
|
In tetrahydrofuran; at 55 - 60 ℃; for 3.5h; Inert atmosphere;
|
|
|
In water; isopropyl alcohol; at -20 - -5 ℃;
|
|
|
In tetrahydrofuran; at 10 - 20 ℃; for 0.666667h; Product distribution / selectivity;
|
|
|
In methanol; at 50 ℃;
|
|
|
In tert-butyl methyl ether; isopropyl alcohol; at 62 - 63 ℃; for 2 - 3h; Product distribution / selectivity;
|
|
|
In methoxybenzene; isopropyl alcohol; at 25 - 85 ℃; for 4.5h; Product distribution / selectivity; Inert atmosphere;
|
|
|
With pyrographite; In methanol; at 50 ℃; for 0.5h; Reflux;
|
|
|
In propan-1-ol; at 0 - 5 ℃; for 0.833333h; Solvent; Temperature; Time; Inert atmosphere;
|
|
|
In ethanol; at 45 ℃; for 2h;
|
|
|
In methanol; dichloromethane; at 25 ℃; for 2h; Inert atmosphere;
|
|
|
In diphenylether; isopropyl alcohol; at 20 - 70 ℃; for 3.5h; Solvent;
|
418.0 g |
|
In water; ethyl acetate; isopropyl alcohol; at 65 - 75 ℃; for 1h; Solvent; Temperature;
|
468 g |
|
In methanol; at 40 ℃; Industrial scale;
|
Ca. 15 kg |
|
In isopropyl alcohol; at 50 ℃; for 1h;
|
4.9 g |

methanesulfonic acid


imatinib

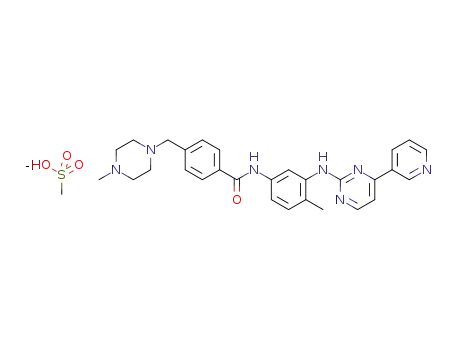
imatinib mesylate
| Conditions | Yield |
|---|---|
|
In isopropyl alcohol; at 20 - 80 ℃; Product distribution / selectivity; Reflux;
|
99.6% |
|
In methanol; at 50 - 55 ℃; for 2.5h; Solvent; Temperature;
|
95% |
|
In methanol; at -10 - 0 ℃; for 0.333333h; Product distribution / selectivity;
|
58% |
|
In ISOPROPYLAMIDE; at 25 - 65 ℃; Product distribution / selectivity;
|
|
|
In N,N-dimethyl acetamide; at 25 - 65 ℃; Product distribution / selectivity;
|
|
|
With potassium mesylate; In water; at 5 - 20 ℃; for 24h; pH=5.2; Product distribution / selectivity;
|
|
|
In water; pH=3 - 3.5;
|

methanesulfonic acid

imatinib
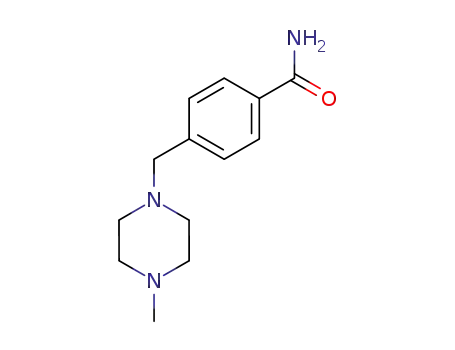
4-((4-methylpiperazin-1-yl)methyl)benzamide
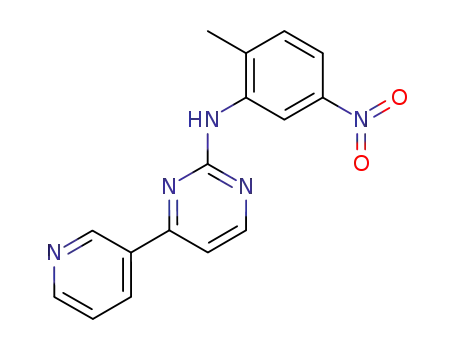
N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine
CAS:143062-84-4
CAS:152459-95-5
CAS:1953-04-4