- Language:English
- English
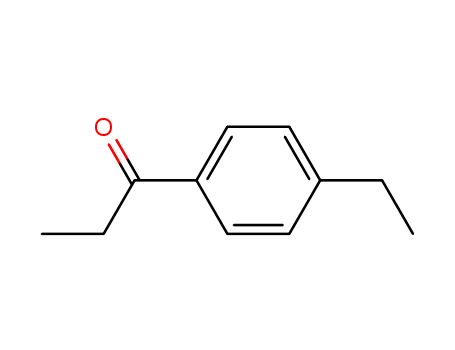

CasNo: 27465-51-6
Molecular Formula: C11H14O
InChI:InChI=1/C11H14O/c1-3-10-4-6-11(7-5-10)8-9(2)12/h4-7H,3,8H2,1-2H3
A selective electrochemical oxidation wa...
Ketones are of great importance in synth...
The invention relates to a method for pr...
A highly efficient acylative cross-coupl...
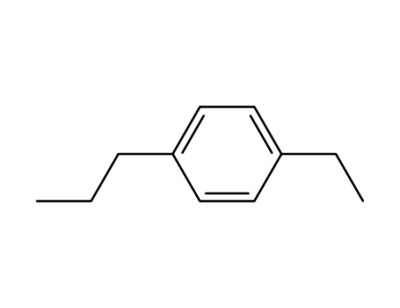
p-(n-propyl)ethylbenzene

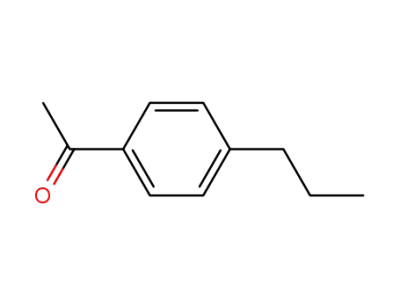
1-(4-propylphenyl)ethan-1-one

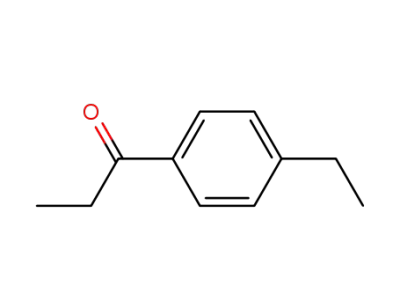
1-(4-ethylphenyl)-1-propanone
| Conditions | Yield |
|---|---|
|
With
pyridine; N-hydroxyphthalimide; tetrabutylammonium tetrafluoroborate; oxygen;
In
2,2,2-trifluoroethanol; acetonitrile;
at 35 ℃;
Overall yield = 65 percent;
Electrolysis;
|

triethyl borane

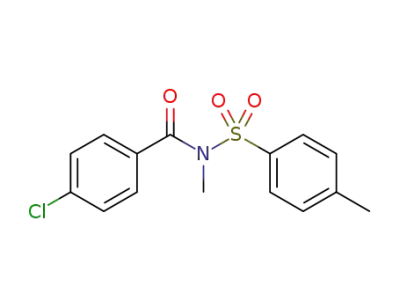
N-methyl-N-tosyl-p-chlorobenzamide


1-(4-ethylphenyl)-1-propanone
| Conditions | Yield |
|---|---|
|
With
[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride; potassium carbonate;
In
tetrahydrofuran; tert-butyl methyl ether;
at 20 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
88% |
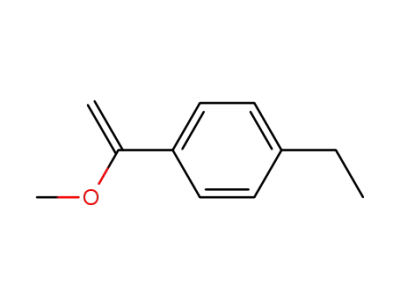
[1-(4-ethyl-phenyl)-vinyl]-methyl ether
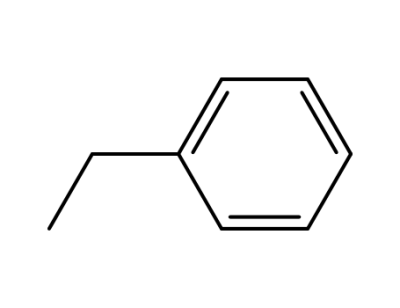
ethylbenzene
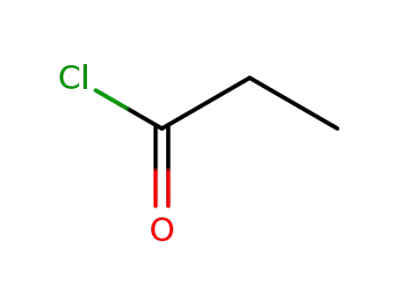
propionyl chloride
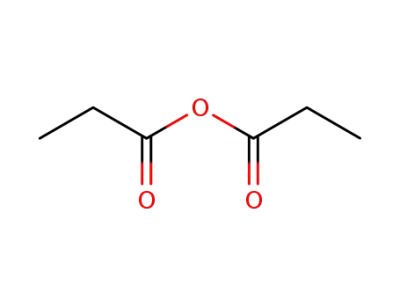
propionic acid anhydride
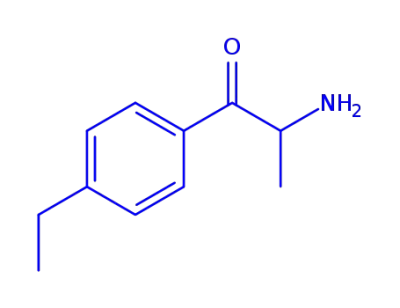
α-Amino-4-ethyl-propiophenon

p-(n-propyl)ethylbenzene
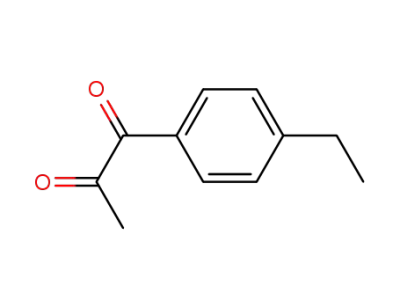
1-(4-ethylphenyl)propane-1,2-dione
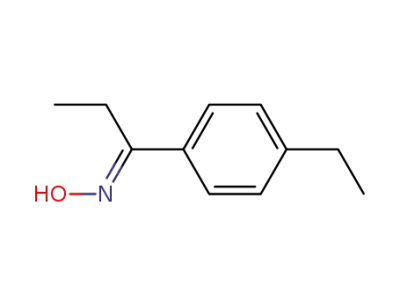
1-(4-ethyl-phenyl)-propan-1-one oxime
CAS:138071-82-6
CAS:57280-22-5
CAS:32804-77-6
CAS:70-70-2