- Language:English
- English
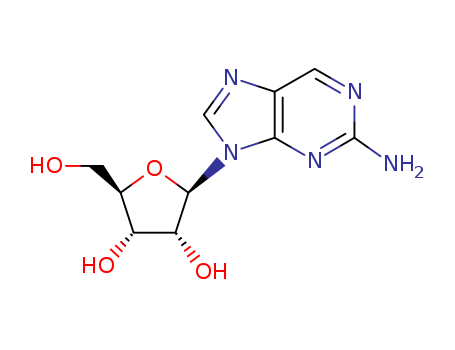

CasNo: 4546-54-7
Molecular Formula: C10H13N5O4
Appearance: Off-white crystalline solid
InChI:InChI=1/C10H13N5O4/c11-10-12-1-4-8(14-10)15(3-13-4)9-7(18)6(17)5(2-16)19-9/h1,3,5-7,9,16-18H,2H2,(H2,11,12,14)
2-Aminopurine (Ap) is a fluorescent nucl...
The Vorbrueggen glycosylation reaction w...
Sodium naphthalenide effects removal of ...
A simple synthesis of 2-aminopurine nucl...
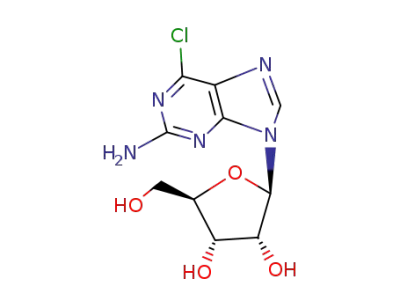
2-Amino-6-chloropurine riboside

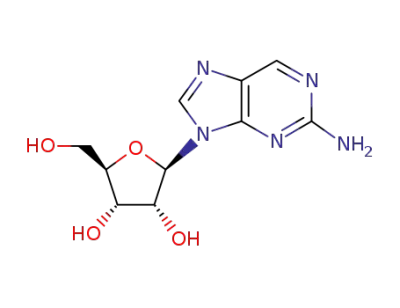
2?amino?9?(β?D?ribofuranosyl)purine
| Conditions | Yield |
|---|---|
|
With
palladium hydroxide, 20 wt% on carbon; ammonium formate;
In
1,4-dioxane; methanol;
for 1h;
Reflux;
|
100% |
|
Multi-step reaction with 3 steps
1: pyridine / 0.5 h
2: sodium naphthalenide / tetrahydrofuran / -60 °C
3: aq. NH3 / methanol / 0.5 h
With
pyridine; ammonium hydroxide; sodium naphthalenide;
In
tetrahydrofuran; methanol;
|
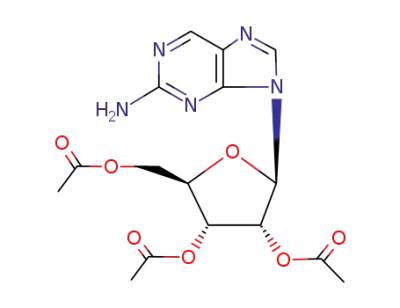
2-amino-9-(2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)purine


2?amino?9?(β?D?ribofuranosyl)purine
| Conditions | Yield |
|---|---|
|
With
ammonia;
In
ethanol;
at 25 ℃;
for 23h;
|
96% |
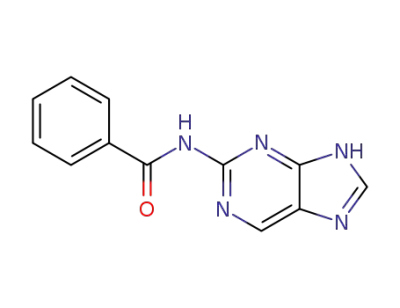
2-benzoylamidopurine

2-amino-9-(2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)purine
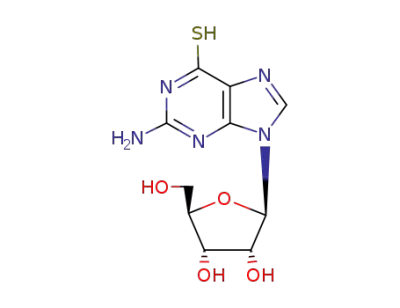
6-mercaptoguanosine
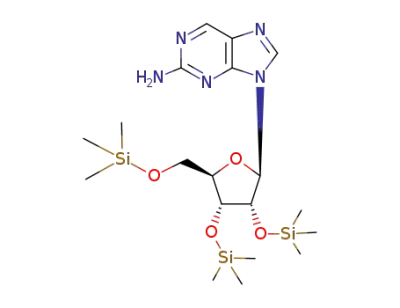
9-((2R,3R,4R,5R)-3,4-Bis-trimethylsilanyloxy-5-trimethylsilanyloxymethyl-tetrahydro-furan-2-yl)-9H-purin-2-ylamine
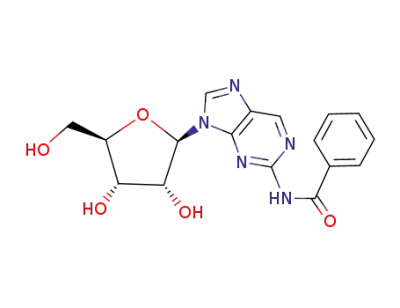
N-benzoyl-9-(β-D-ribofuranosyl)-9H-purin-2-amine
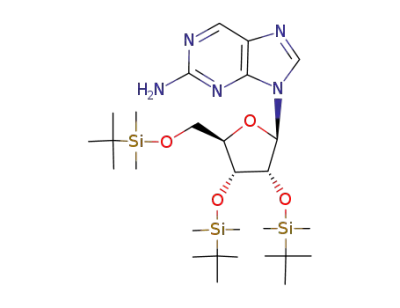
2',3',5'-tri-O-tert-butyldimethylsilyl-9-(β-D-ribofuranosyl)-2-amino-purine
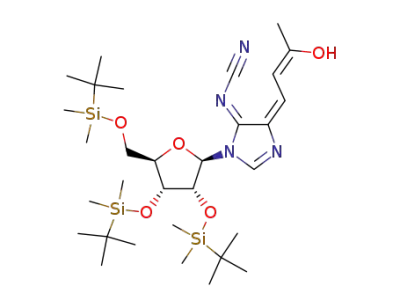
3-[(2R,3R,4R,5R)-3,4-Bis-(tert-butyl-dimethyl-silanyloxy)-5-(tert-butyl-dimethyl-silanyloxymethyl)-tetrahydro-furan-2-yl]-5-[(Z)-3-hydroxy-but-2-en-(E)-ylidene]-3,5-dihydro-imidazol-(4E)-ylidene-cyanamide
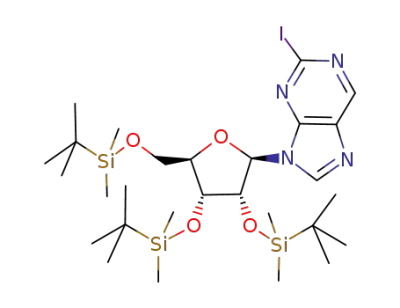
2-Iodo-9-<2,3,5-tri-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl>purine
CAS:138071-82-6
CAS:38083-17-9
CAS:64-02-8
CAS:106-28-5