- Language:English
- English


CasNo: 7236-57-9
Molecular Formula: C10H14N2O4S
|
General Description |
4-thio-thymidine is a chemical analog of the nucleoside, thymidine, which is commonly found in DNA. Thymidine, 4-thio- has a sulfur atom replacing an oxygen atom in the thymidine molecule, which gives it unique photo-reactive properties. It is often used in research as a molecular probe to study the dynamics of DNA and RNA due to its ability to form covalent bonds upon exposure to UV light. It is efficiently incorporated into DNA and its absorbance and fluorescence properties enable it to be tracked and visualized in biological systems. |
InChI:InChI=1/C10H14N2O4S/c1-5-3-12(10(15)11-9(5)17)8-2-6(14)7(4-13)16-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,17)/t6-,7+,8+/m0/s1
4 - Thio-deoxythymidine derivatives and ...
The interaction of 4-thiothymidine (S4Td...
Unambiguous characterization of 5-substi...
5-Substituted-4-thio-2'-deoxyuridine nuc...
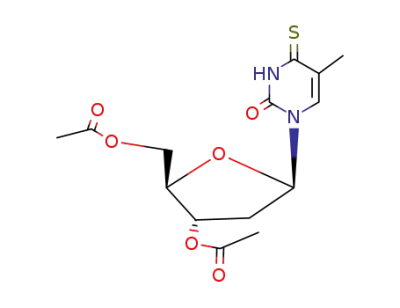
3',5'-di-O-acetyl-4-thiothymidine

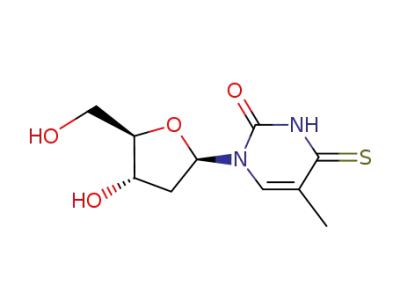
4-thiothymidine
| Conditions | Yield |
|---|---|
|
With
ammonia;
In
methanol;
|
|
|
With
ammonia;
In
methanol;
at 20 ℃;
for 4.5h;
|
|
|
With
methanol;
at 20 ℃;
Alkaline conditions;
|
22.6 g |
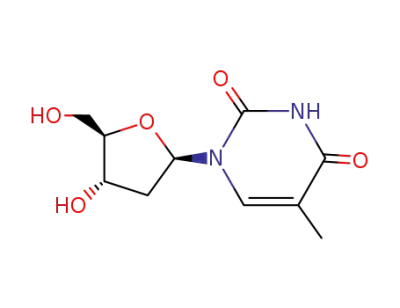
thymidine


4-thiothymidine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: pyridine
2: aqueous pyridine; P2S5
3: methanol. sodium methylate
With
pyridine; tetraphosphorus decasulfide; sodium methylate;
|
|
|
Multi-step reaction with 4 steps
1.1: triethylamine / tetrahydrofuran / 2.5 h / Cooling with ice
2.1: triethylamine; trichlorophosphate / acetonitrile / 0 °C
2.2: 16 h / 20 °C
3.1: acetonitrile / Cooling with ice
4.1: hydrogenchloride; water / tetrahydrofuran / 20 h / 20 °C / pH 3
With
hydrogenchloride; water; triethylamine; trichlorophosphate;
In
tetrahydrofuran; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: pyridine
2: tetraphosphorus decasulfide / 1,4-dioxane
3: ammonia / methanol
With
pyridine; tetraphosphorus decasulfide; ammonia;
In
1,4-dioxane; methanol;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / 5 h / 0 °C
2: tetraphosphorus decasulfide / 1,4-dioxane / 1.5 h / 106 °C
3: ammonia / methanol / 4.5 h / 20 °C
With
pyridine; tetraphosphorus decasulfide; ammonia;
In
1,4-dioxane; methanol;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / 20 °C
2: Lawessons reagent / toluene / 4 h / 97 °C
3: methanol / 20 °C / Alkaline conditions
With
Lawessons reagent; pyridine; methanol;
In
toluene;
|
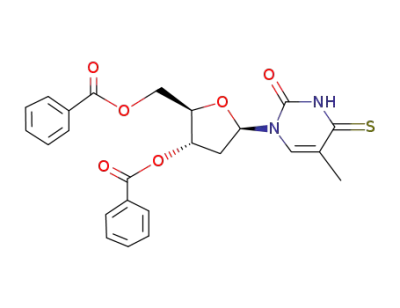
O3',O5'-dibenzoyl-4-thio-thymidine
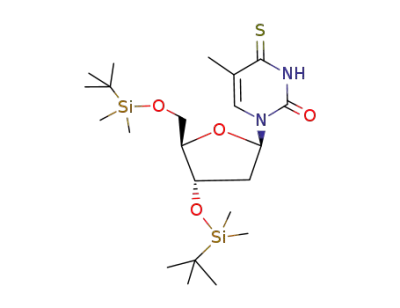
1-[(2R,4S,5R)-4-(tert-Butyl-dimethyl-silanyloxy)-5-(tert-butyl-dimethyl-silanyloxymethyl)-tetrahydro-furan-2-yl]-5-methyl-4-thioxo-3,4-dihydro-1H-pyrimidin-2-one
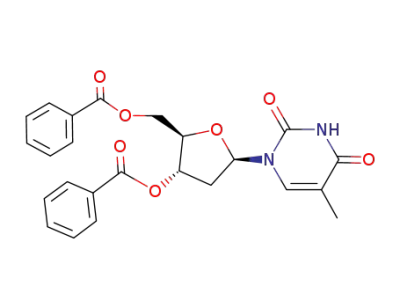
3',5'-di-O-benzoylthymidine
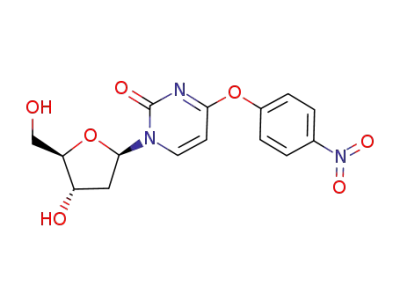
4-(p-nitrophenoxy)-1-(β-D-2-deoxyribofuranosyl)pyrimidin-2(1H)-one
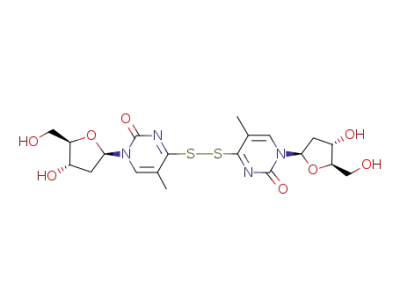
di-thymidin-4-yl disulfide
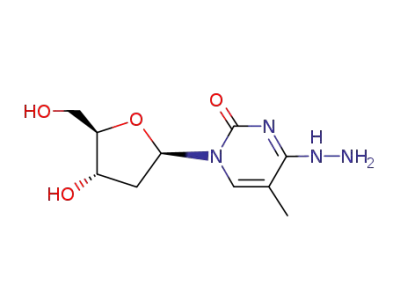
1-(2-deoxy-β-D-erythro-pentofuranosyl)-4-hydrazino-5-methylpyrimidin-2(1H)-one
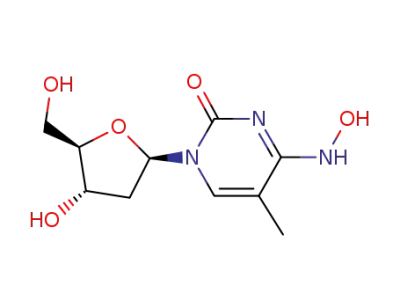
5-methyl-2'-deoxy-N4-hydroxycytidine
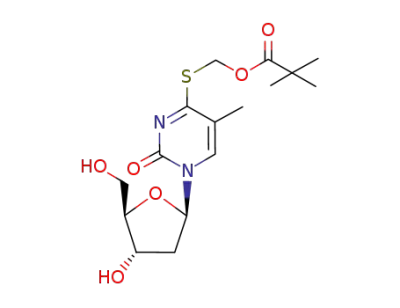
4-S-pivaloyloxymethyl-4-thiothymidine
CAS:143062-84-4
CAS:71675-87-1
CAS:19356-17-3
CAS:134404-52-7