- Language:English
- English
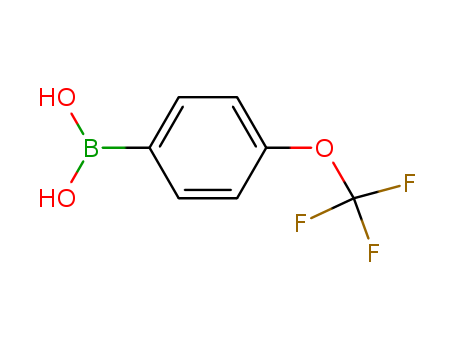

CasNo: 139301-27-2
Molecular Formula: C7H6BF3O3
Appearance: white to beige crystalline powder
|
Application |
4-Trifluoromethoxyphenylboronic acid can reactant involved in the synthesis of biologically active molecules including:Lactate dehydrogenase inhibitors for use against cancer cell proliferationNitro-phenoxybenzoic acid derivatives for PAI-1 inhibitionPA-824 analogs for use as antituberculosis drugsModulators of survival motor neuron proteinReactant involved in addition reactions and cross-coupling reactions including Suzuki-Miyaura cross-coupling |
InChI:InChI=1/C7H6BF3O3/c9-7(10,11)14-6-3-1-5(2-4-6)8(12)13/h1-4,12-13H
In this study, we developed a simple tra...
We report herein a simple, metal- and ad...
The present invention has its object to ...
The present invention provides, among ot...
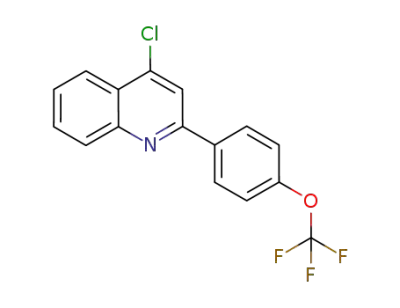
4-chloro-2-(4-trifluoromethoxy-phenyl)-quinoline

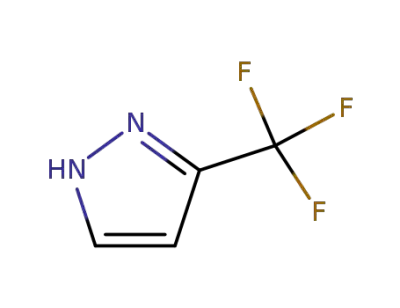
3-(trifluoromethyl)pyrazole

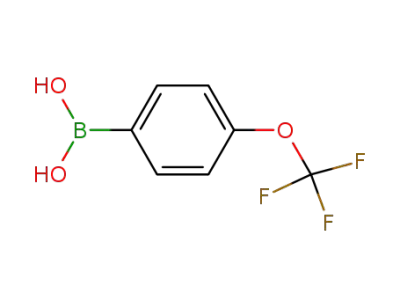
4-trifluoromethoxyphenylboronic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 80 - 100 ℃;
for 14h;
|
52% |
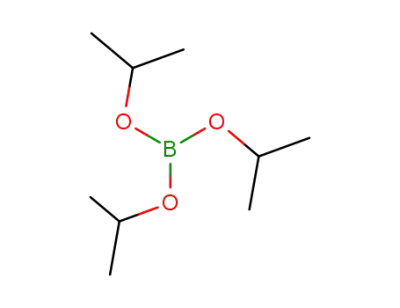
Triisopropyl borate

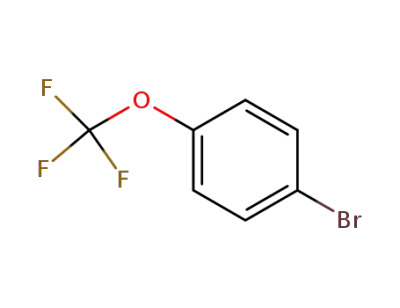
1-bromo-4-(trifluoromethoxy)benzene


4-trifluoromethoxyphenylboronic acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; n-butyllithium; sodium chloride;
In
tetrahydrofuran; n-heptane;
|
1.306 kg (90.4%) |
|
With
hydrogenchloride; magnesium;
In
tetrahydrofuran;
|

1-bromo-4-(trifluoromethoxy)benzene

3-(trifluoromethyl)pyrazole

Triisopropyl borate
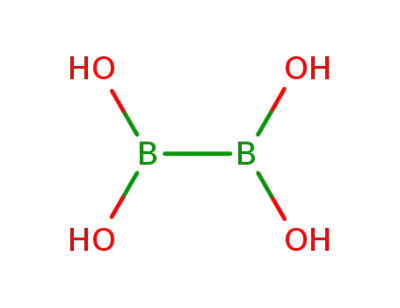
tetrahydroxydiboron
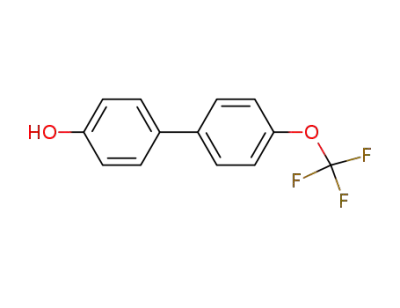
4-(4'-trifluoromethoxy phenyl)-phenol
5-(4-trifluoromethoxyphenyl) furan-2-formaldehyde
CAS:480424-84-8
CAS:5116-24-5
CAS:2149-76-0