- Language:English
- English
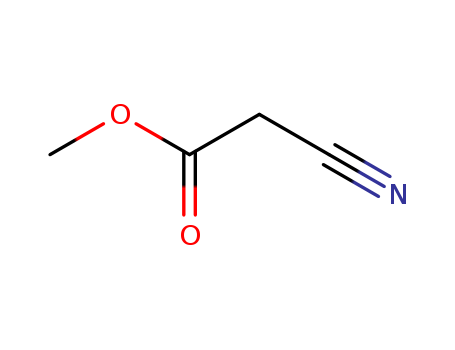

CasNo: 105-34-0
Molecular Formula: C4H5NO2
Appearance: clear colorless to very slighlty yellow liquid
|
Purification Methods |
Purify the ester by shaking with 10% Na2CO3 solution, wash well with water, dry with anhydrous Na2SO4, and distil it. [Beilstein 2 H 584, 2 I 253, 2 II 530, 2 III 1628, 2 IV 1889.] |
|
General Description |
Methyl cyanoacetate is a versatile chemical reagent used in various synthetic pathways, including the formation of hetarylidene derivatives through condensation reactions with aldehydes, as well as in multicomponent reactions to produce biologically active pyrimidinones and their salts. It serves as an active methylene compound in nucleophilic substitution reactions, enabling the synthesis of diverse heterocyclic systems such as thiapentalenes. Its utility is highlighted in efficient one-pot syntheses, offering advantages like high bond-forming efficiency and atom economy, making it valuable in heterocyclic chemistry and materials science. |
InChI:InChI:1S/C4H5NO2/c1-7-4(6)2-3-5/h2H2,1H3
Primary aliphatic and aromatic nitriles ...
A Ti4+-exchanged montmorillonite (Ti4+-m...
-
Activation of carbonyl moiety is one of ...
-
-
Here we report a novel family of crystal...
One-pot borontrifluoride etherate promot...
N-halosuccinimides (NXSs) are well-known...
Aryl/alkyl cyanides were quickly convert...
-
Recent research shows high potential for...
A new porous zinc phosphonate material (...
-
The synthesis of nitrile under mild cond...
The invention discloses a bruton's tyros...
The invention discloses a green synthesi...
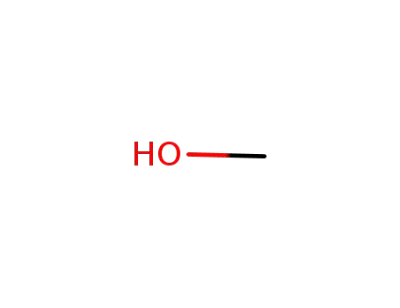
methanol

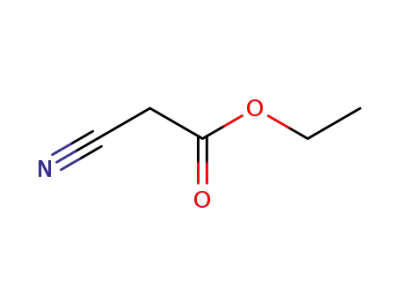
ethyl 2-cyanoacetate

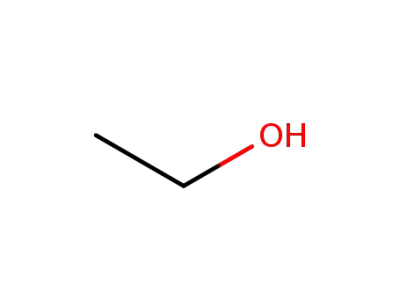
ethanol

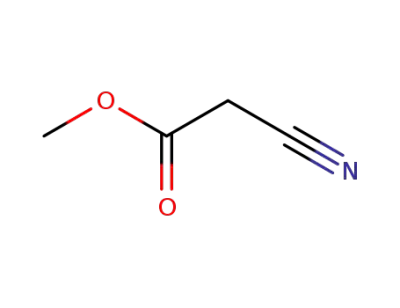
methyl 2-cyanoacetate
| Conditions | Yield |
|---|---|
|
methanol; ethyl 2-cyanoacetate;
at 59.84 ℃;
for 0.25h;
With
hybrid supermicroporous iron(III) phosphonate nanoparticle (HFeP-1-3);
at 59.84 ℃;
for 6h;
Reagent/catalyst;
|
85.6% |

methanol

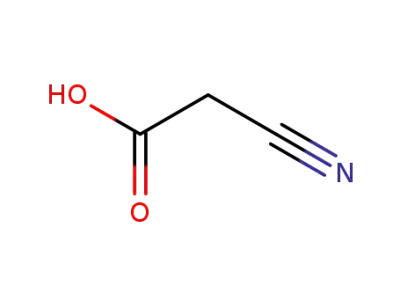
cyanoacetic acid


methyl 2-cyanoacetate
| Conditions | Yield |
|---|---|
|
With
N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine;
In
dichloromethane;
for 1h;
Ambient temperature;
|
97% |
|
With
N-Bromosuccinimide;
at 70 ℃;
for 20h;
|
97% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;
at 70 ℃;
for 20h;
|
97% |
|
With
sulfuric acid; sulfur trioxide;
at 60 - 82 ℃;
for 4h;
|
93.2% |
|
Amberlyst 15;
In
methanol;
for 7h;
Ambient temperature;
|
85% |
|
With
sulfuric acid;
at 70 ℃;
for 12h;
|
69% |
|
With
sulfuric acid;
unter Entfernen des entstehenden Wassers;
|
|
|
With
chloro-trimethyl-silane; 2,2-dimethoxy-propane;
for 24h;
Yield given;
Ambient temperature;
|
|
|
With
Ti(4+) montmorillonite;
for 20h;
Heating;
|
|
|
With
dmap; dicyclohexyl-carbodiimide;
In
dichloromethane;
at 25 ℃;
for 2h;
|
|
|
With
sulfuric acid;
|
|
|
With
sulfuric acid;
Reflux;
|
|
|
With
dicyclohexyl-carbodiimide;
In
acetonitrile;
at 20 ℃;
for 2h;
|

methanol
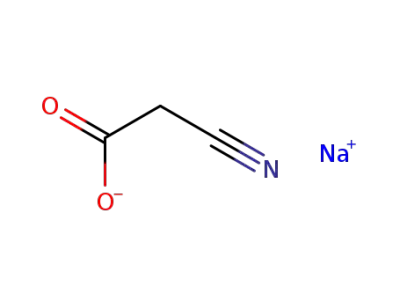
sodium cyanoacetate
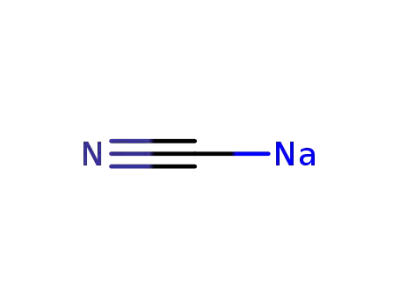
sodium cyanide

methyl chloroacetate
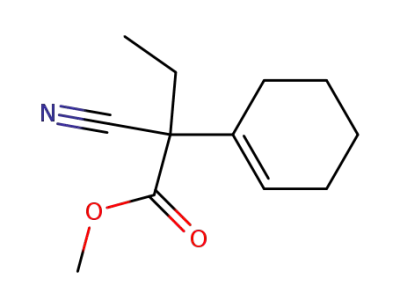
2-cyano-2-cyclohex-1-enyl-butyric acid methyl ester

3-cyano-2-hydroxy-2-phenyl-succinic acid dimethyl ester
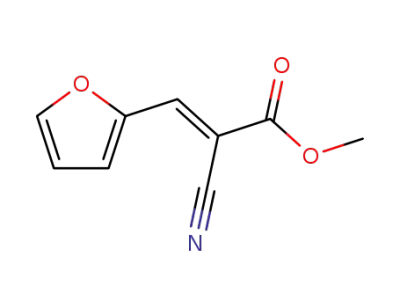
methyl (E)-2-cyano-3-(2-furyl)-2-propenoate
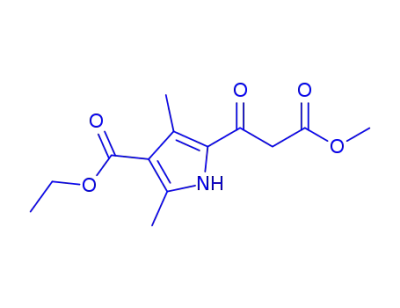
3-(4-ethoxycarbonyl-3,5-dimethyl-pyrrol-2-yl)-3-oxo-propionic acid methyl ester
CAS:138071-82-6
CAS:38083-17-9
CAS:72957-37-0
CAS:2757690-14-3