- Language:English
- English
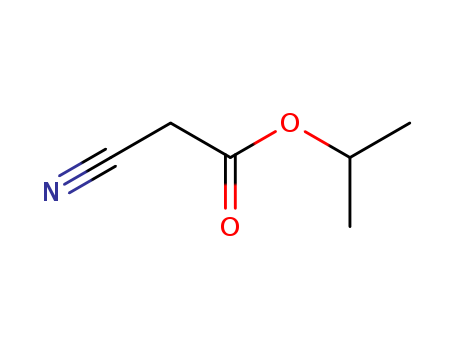

CasNo: 13361-30-3
Molecular Formula: C6H9NO2
|
Synthesis Reference(s) |
Synthesis, p. 138, 1982 DOI: 10.1055/s-1982-29718 |
InChI:InChI=1/C6H9NO2/c1-5(2)9-6(8)3-4-7/h5H,3H2,1-2H3
In this work, a new Fe3O4/AlFe/Te nanoco...
Compound 1 (Figure 1), labeled with carb...
Novel 2-amino-1,4-dihydropyridine deriva...
-
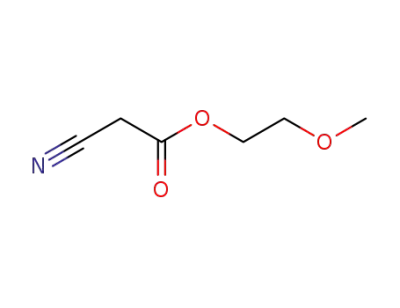
2-methoxyethyl cyanoacetate

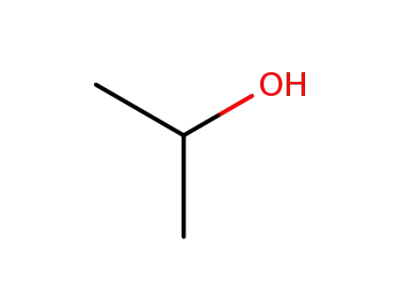
isopropyl alcohol

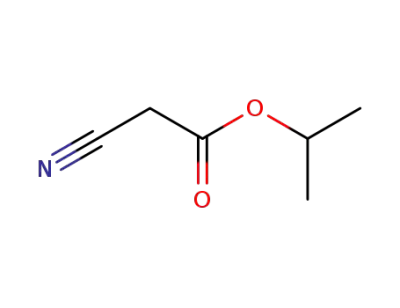
isopropyl cyanoacetate
| Conditions | Yield |
|---|---|
|
With
titanium(IV) tetraethanolate;
at 82 ℃;
for 6h;
|
77% |

cyanoacetic acid


isopropyl alcohol


isopropyl cyanoacetate
| Conditions | Yield |
|---|---|
|
With
dicyclohexyl-carbodiimide;
In
tetrahydrofuran;
at 55 ℃;
|
80% |
|
With
Fe3O4/AlFe/Te nanocomposite;
In
neat (no solvent);
for 1h;
Reflux;
|

2-methoxyethyl cyanoacetate

isopropyl alcohol

cyanoacetic acid
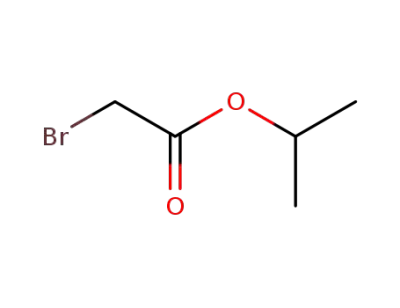
isopropyl 2-bromoacetate
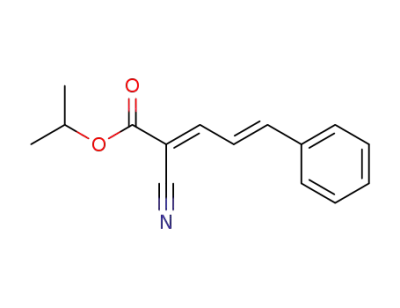
isopropyl (2E,4E)-2-cyano-5-phenylpenta-2,4-dienoate
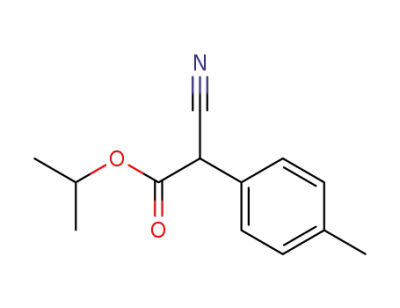
Cyano-p-tolyl-acetic acid isopropyl ester

cyano<5-fluoro-1-(4-methylbenzyl)-2-oxo-2,3-dihydroindol-3-ylidene>acetic acid isopropyl ester
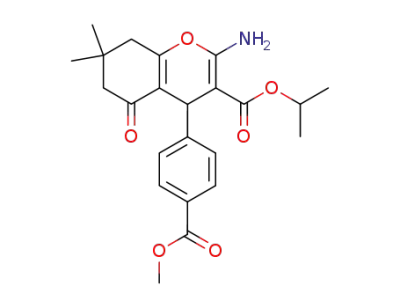
2-amino-3-isopropyloxycarbonyl-4-(4-methoxycarbonylphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyran
CAS:138071-82-6
CAS:38083-17-9
CAS:302776-68-7
CAS:41294-56-8