- Language:English
- English
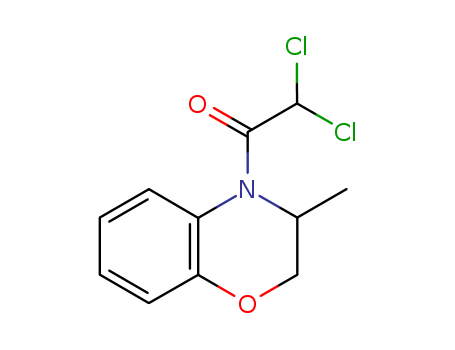

CasNo: 98730-04-2
Molecular Formula: C11H11Cl2NO2
Appearance: transparent oil liquid
|
Uses |
Benoxacor, a chiral herbicide safener for S-metolachlor, has been detected in streams. The application of benoxacor alongside the herbicide promotes rapid in vivo transformation of the herbicide to less toxic metabolites within cereal crops, but not weed species, thus protecting the target crop from herbicidal damage. |
|
Flammability and Explosibility |
Nonflammable |
|
Metabolic pathway |
In cell suspension cultures of corn (Zea mays) with 14C-benoxacor, benoxacor is rapidly metabolized to six detectable metabolites within 0.5 h. Twelve metabolites are detected in extracts from the treated cells for 24 h. Of the three predominant metabolites present, two metabolites are the catabolic formylcarboxamide and carboxycarboxamide derivatives of benoxacor. The third one is the mono glutathione conjugate of benoxacor. This metabolite consists of a single glutathione molecule linked via the cysteinyl sulfhydryl group to the N-dichloroacetyl a- carbon of benoxacor. A catabolic a-hydroxyacetamide derivative is detected as well as its amino acid conjugates either containing glutathione residue or presumably derived from the glutathione residue. A disaccharide conjugate is identified as S-(O-diglycoside)glutathione conjugate. |
InChI:InChI=1/C11H11Cl2NO2/c1-7-6-16-9-5-3-2-4-8(9)14(7)11(15)10(12)13/h2-5,7,10H,6H2,1H3
Results showed that benoxacor had negative effects on hatchability, malformations, and mortality. Compared to either individual enantiomer, embryos exposed to Rac-benoxacor had higher acute and developmental toxicities, glutathione S-transferase (GST) and glutathione peroxidase (GPx) enzyme activities, and nrf 2 expression levels.
A three-step sequence was developed for ...
A glutathione S-transferase (GST) isozyme from maize (Zea mays Pioneer hybrid 3906) treated with the dichloroacetamide herbicide safener benoxacor (CGA-154281) was purified to homogeneity and partially characterized.
Here we investigate the enantioselective metabolism of benoxacor in human subcellular fractions. Our results indicate that human hepatic microsomal and cytosolic enzymes enantioselectively metabolize benoxacor, a fact that needs to be considered when investigating human exposures and toxicities of benoxacor.
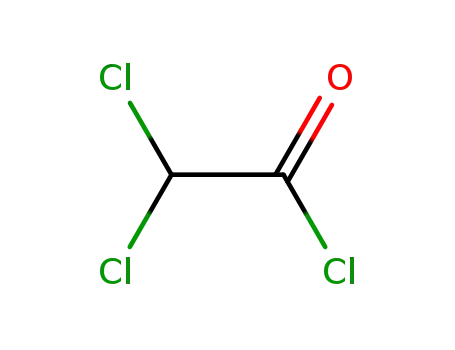
dichloroacethyl chloride

![(RS)-3-methyl-3,4-dihydro-2H-[1,4]benzoxazine](/upload/2023/6/317a7dd5-6579-4870-b597-f3514feee538.png)
(RS)-3-methyl-3,4-dihydro-2H-[1,4]benzoxazine

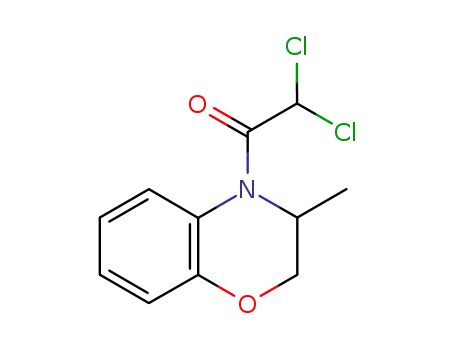
4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine
| Conditions | Yield |
|---|---|
|
In 5,5-dimethyl-1,3-cyclohexadiene; for 1.5h; Solvent; Reflux; Large scale;
|
96.9% |
|
With phosphoric acid; sodium hydroxide; In water; toluene; at 40 - 80 ℃; for 2.5h; pH=2 - 3; Industry scale;
|
91% |
|
With sodium carbonate; In benzene; at 20 ℃; for 2h;
|
66% |
|
With sodium carbonate; In di-isopropyl ether; water; ethyl acetate; benzene;
|
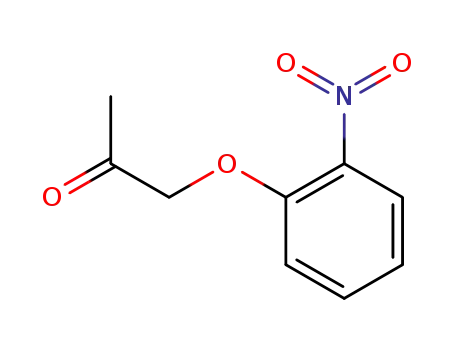
1-(2-nitrophenoxy)-propan-2-one


4-dichloroacetyl-3,4-dihydro-3-methyl-2H-1,4-benzoxazine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: platinum on activated charcoal; hydrogen / toluene; isopropyl alcohol / 10 h / 60 °C / 11251.1 Torr
2: sodium carbonate / benzene / 2 h / 20 °C
With platinum on activated charcoal; hydrogen; sodium carbonate; In isopropyl alcohol; toluene; benzene;
|

dichloroacethyl chloride
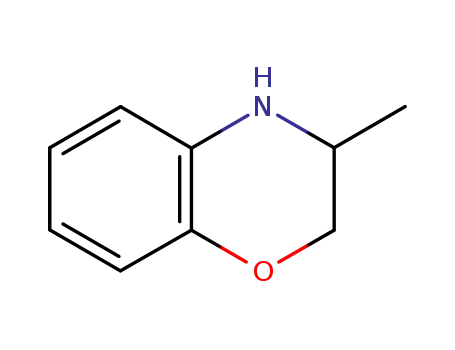
(RS)-3-methyl-3,4-dihydro-2H-[1,4]benzoxazine

1-(2-nitrophenoxy)-propan-2-one
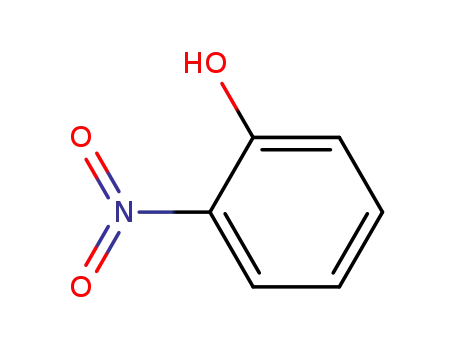
2-hydroxynitrobenzene
CAS:38083-17-9
CAS:120011-70-3
CAS:144171-61-9
CAS:17162-29-7