- Language:English
- English
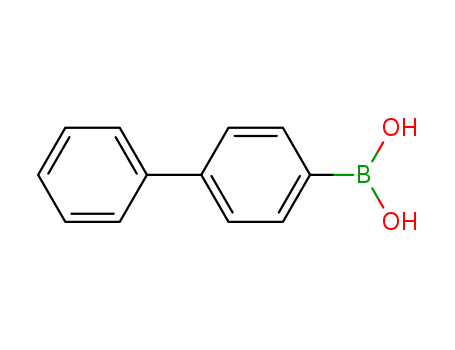

CasNo: 5122-94-1
Molecular Formula: C12H11BO2
Appearance: Solid
InChI:InChI=1/C12H11BO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,14-15H
(Figure Presented) In best order: Nanoag...
The reaction between di-2-thienyl ketone...
The invention provides a nitrogen-contai...
Asymmetric 1,4-addition reactions with β...
In this study, we developed a simple tra...
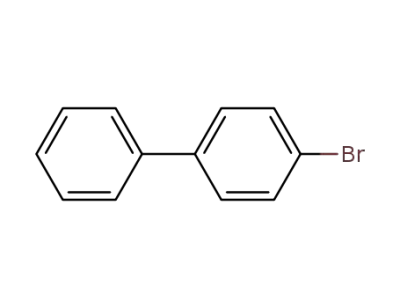
4-bromo-1,1'-biphenyl

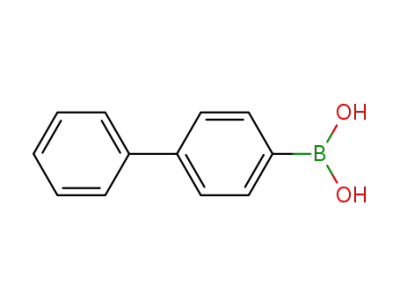
4-biphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1.5h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 4.5h;
With
water;
In
tetrahydrofuran; hexane; ethyl acetate;
|
84% |
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2.5h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
76% |
|
4-bromo-1,1'-biphenyl;
With
magnesium;
In
tetrahydrofuran;
for 4h;
Heating;
With
Trimethyl borate;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
67% |
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexanes;
at -80 ℃;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexanes;
at -80 - 20 ℃;
Inert atmosphere;
With
hydrogenchloride;
In
tetrahydrofuran; hexanes; water;
at 20 ℃;
Inert atmosphere;
|
64% |
|
Multistep reaction;
(i) Mg, (ii) B(OMe)3, (iii) H2SO4;
|
|
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 6h;
|
|
|
4-bromo-1,1'-biphenyl;
With
magnesium;
In
tetrahydrofuran;
at 50 ℃;
for 2h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -60 - 15 ℃;
for 2h;
Further stages.;
|
|
|
Multi-step reaction with 2 steps
1.1: n-BuLi / tetrahydrofuran / 0.42 h / -78 °C
1.2: tetrahydrofuran / -78 - 20 °C
2.1: aq. HCl / 3 h / pH 6 - 7
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran;
|
|
|
4-bromo-1,1'-biphenyl;
With
magnesium;
In
tetrahydrofuran;
With
Trimethyl borate;
With
hydrogenchloride;
Further stages.;
|
|
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
diethyl ether; hexane; toluene;
at -64 ℃;
for 2.5h;
With
Trimethyl borate;
In
diethyl ether; hexane; toluene;
at 20 ℃;
for 12.25h;
With
hydrogenchloride; water;
In
diethyl ether; hexane; toluene;
at 0 - 10 ℃;
|
|
|
With
tetrahydroxydiboron; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N-ethyl-N,N-diisopropylamine; triphenylphosphine;
In
ethanol;
at 20 ℃;
for 6h;
Inert atmosphere;
Sealed tube;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 1 h / -78 - 0 °C / Inert atmosphere
1.2: 12 h / -78 - 20 °C / Inert atmosphere
2.1: hydrogenchloride / tetrahydrofuran / 0.5 h / Inert atmosphere
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran;
|
|
|
Multi-step reaction with 2 steps
1.1: magnesium; lithium chloride / Inert atmosphere; Schlenk technique
1.2: Inert atmosphere; Schlenk technique
2.1: copper(I) iodide-lithium chloride / tetrahydrofuran / 20 °C / Schlenk technique; Inert atmosphere
With
copper(I) iodide-lithium chloride; magnesium; lithium chloride;
In
tetrahydrofuran;
|
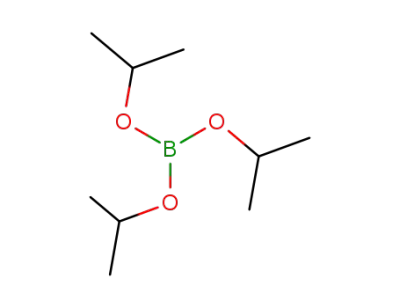
Triisopropyl borate

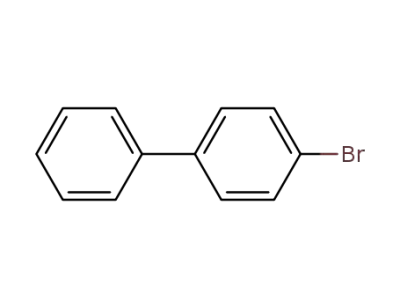
4-bromo-1,1'-biphenyl


4-biphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-bromo-1,1'-biphenyl;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 ℃;
for 0.5h;
Triisopropyl borate;
In
diethyl ether; hexane;
at -78 - 20 ℃;
|
74% |
|
With
n-butyllithium;
In
tetrahydrofuran;
|
1.53 g (90%) |
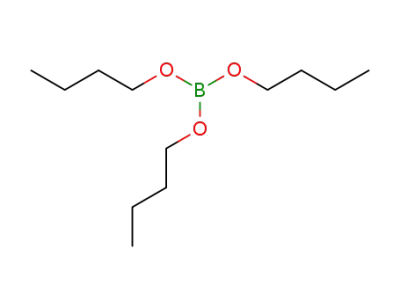
boric acid tributyl ester
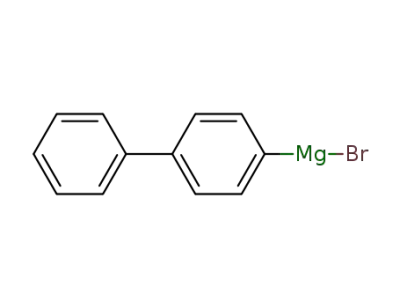
4-biphenylylmagnesium bromide
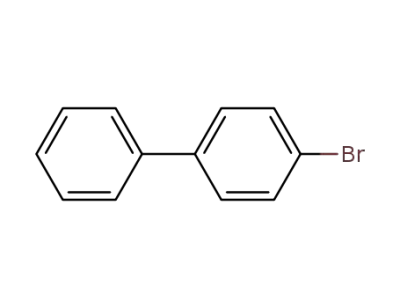
4-bromo-1,1'-biphenyl

4-iodo-biphenyl
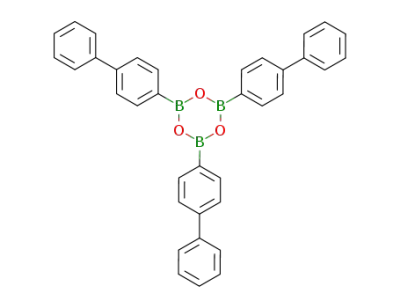
2,4,6-tris([1,1'-biphenyl]-4-yl)boroxine
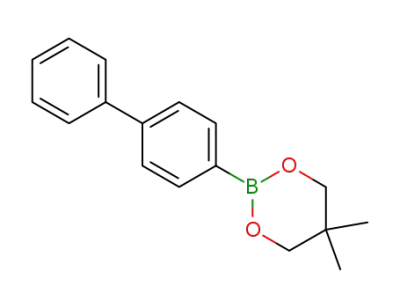
2-([1,1'-biphenyl]-4-yl)-5,5-dimethyl-1,3,2-dioxaborinane
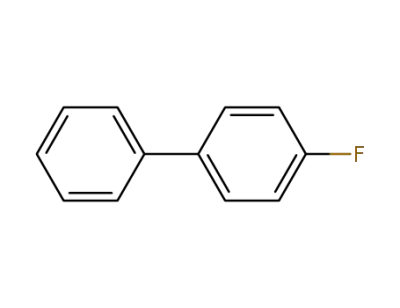
4-fluoro-biphenyl
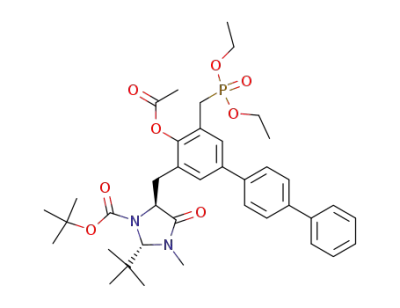
(2S,5S)-5-[4-Acetoxy-5-(diethoxy-phosphorylmethyl)-[1,1';4',1'']terphenyl-3-ylmethyl]-2-tert-butyl-3-methyl-4-oxo-imidazolidine-1-carboxylic acid tert-butyl ester
CAS:1953-04-4
CAS:20826-04-4
CAS:2133-80-4
CAS:5720-05-8