- Language:English
- English


CasNo: 122775-35-3
Molecular Formula: C8H11BO4
Appearance: white to light beige powder and granules
InChI:InChI=1/C8H11BO4/c1-12-7-4-3-6(9(10)11)5-8(7)13-2/h3-5,10-11H,1-2H3
A Sandmeyer borylation of arylamines via...
Five libraries of natural and synthetic ...
A catalytic domino reduction–imine forma...
An asymmetric methanolysis of glutaric a...
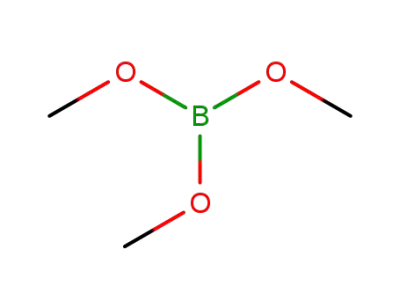
Trimethyl borate

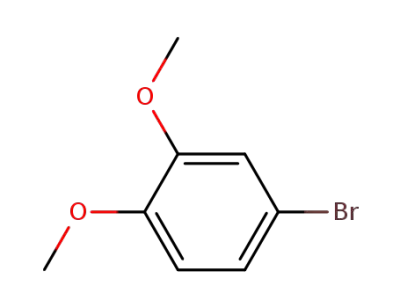
4-Bromoveratrole

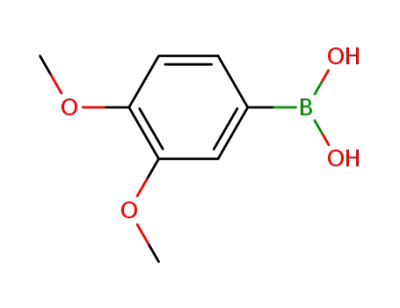
3,4-Dimethoxyphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-Bromoveratrole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 23 ℃;
for 4h;
In
tetrahydrofuran; water;
at 0 - 23 ℃;
pH=2;
|
87% |
|
With
Mg;
In
tetrahydrofuran;
Mg (reflux), THF, B(OCH)3, bromobenzene-compound (-10°C) or n-butyllithium, B(OCH3)3, -78°C, THF;
|
78% |
|
4-Bromoveratrole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 17h;
Inert atmosphere;
With
hydrogenchloride;
In
tetrahydrofuran; hexane; water;
at 20 ℃;
for 2h;
pH=1;
Inert atmosphere;
|
64% |
|
4-Bromoveratrole;
With
tert.-butyl lithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
|
|
|
4-Bromoveratrole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 25 ℃;
Inert atmosphere;
|

4-Bromoveratrole


3,4-Dimethoxyphenylboronic acid
| Conditions | Yield |
|---|---|
|
4-Bromoveratrole;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Inert atmosphere;
|
78% |
|
4-Bromoveratrole;
With
tert.-butyl lithium;
In
tetrahydrofuran;
at -78 ℃;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Further stages.;
|
39% |
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 2 h / -78 °C / Inert atmosphere
1.2: -78 - 20 °C / Inert atmosphere
2.1: hydrogenchloride / tetrahydrofuran; water / -20 - 20 °C / Inert atmosphere
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran; water;
|
|
|
4-Bromoveratrole;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
Schlenk technique;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 5h;
Schlenk technique;
|

Trimethyl borate

4-Bromoveratrole
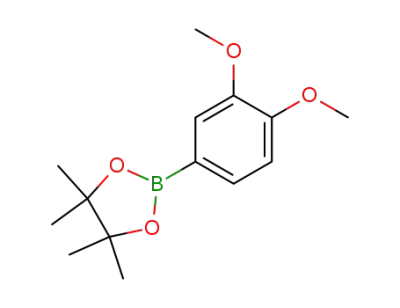
2-(3,4-dimethoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
1,2-dimethoxybenzene
2-methoxy-4-(3,4-dimethoxyphenyl)-3-pivaloylaminopyridine
3-(3,4-dimethoxyphenyl)-4H-chromen-4-one
4-(3,4-Dimethoxyphenyl)-8-methoxy-5-nitroquinoline
7-(3,4-Dimethoxy-phenyl)-1H-indole
CAS:1953-04-4
CAS:20826-04-4
CAS:49557-75-7
CAS:70-00-8